Abstract
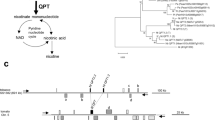
Putrescine N-methyltransferase (PMT) catalyzes the first committed step of nicotine biosynthesis, converting putrescine into N-methylputrescine. A variety of chemical, environmental, and developmental cues have been implicated in its regulation. Here we have examined the differential expression of β-glucuronidase (GUS) transgenes under the control of the transcriptional regulatory sequences of four distinct members of the NtPMT gene family from tobacco (Nicotiana tabacum L.). BY-2 cell cultures expressing various NtPMT promoter-GUS constructs were examined for their response to treatment with various combinations of methyl jasmonate (MeJA), auxin (AUX), and ethylene (ETH). All four NtPMT gene promoters examined were inducible by MeJA, although the extent of the induction varied dramatically, with the NtPMT1a promoter being the most responsive. High AUX levels in the cell growth media repressed NtPMT::GUS transgene expression and inhibited their MeJA-induced transcription. Treatment of BY-2 cells with ETH alone did not result in a significant alteration in NtPMT::GUS expression. However, similar to AUX, ETH treatment led to the suppression of MeJA-induced transcription. Detailed deletion analysis of the NtPMT1a gene promoter showed that as little as 111 bp upstream of the transcriptional start site were sufficient to confer MeJA-responsiveness. Deletion of a conserved G-box element (GCACGTTG) at −103 to −96 bp completely abolished MeJA-responsiveness. Further mutagenesis studies revealed that in addition to a functional G-box, MeJA-responsiveness of the NtPMT1a promoter also required a TA-rich region and a GCC-motif (TGCGCCC) located at −80 to −69 bp and −62 to −56 bp relative to the start site, respectively. A synthetic G-box tetramer (4 X syn G-box) fused to a −83 bp fragment from the NtPMT1apromoter (containing the TA-rich region, GCC-box, and TATA-box) displayed a 30-fold induction by MeJA treatment, whereas when the 4 X syn G-box was fused to a minimal (−46 bp) promoter fragment derived from the CaMV 35S gene, no induction by MeJA treatment was detected. Our results indicate that multiple intersecting signal transduction pathways and different transcriptional regulatory factors are involved in mediating JA-responsiveness of NtPMT expression in tobacco.
Similar content being viewed by others
References
Akehurst, B.C. 1981. The growth, plant structure and genetics. In: D. Rhind and G. Wrigley (Eds.) Tobacco, Longman Press, London, pp. 45–95.
An, G. 1985. High efficiency transformation of cultured tobacco cells. Plant Physiol. 79: 568–570.
Baldwin, I.T. 1999. Inducible nicotine production in native Nicotiana as an example of phenotypic plasticity. J. Chem. Ecol. 25: 3–30.
Baldwin, I.T. and Prestin, C.A. 1999. The eco-physiological complexity of plant responses to insect herbivores. Planta 208: 137–145.
Baldwin, I.T., Schmelz, E.A. and Ohnmeiss, T.E. 1994. Woundinduced changes in root and shoot jasmonic acid pools correlate with induced nicotine synthesis in Nicotiana sylvestris Spegazzini and Comes. J. Chem. Ecol. 20: 2139–2157.
Baldwin, I.T., Schmelz, E.A. and Zhang, Z.P. 1996. Effects of octadecanoic metabolites and inhibitors on induced nicotine accumulation in Nicotiana sylvestris. J. Chem. Ecol. 22: 61–74.
Baldwin, I.T., Zhang, Z.P., Diab, N., Ohnmeiss, T.E., McCloud, E.S., Lynds, G.Y. and Schmelz, E.A. 1997. Quantification, correlations, and manipulations of woundinduced changes in jasmonic acid and nicotine in Nicotiana sylvestris. Planta 201: 397–404.
Buck, M.J. and Atchley, W.R. 2003. Phylogenetic analysis of plant basic helix-loop-helix proteins. J. Mol. Evol. 56: 742–750.
Buttner, M. and Singh, K.B. 1997. Arabidopsis thaliana ethylene-responsive element binding protein (AtEBP), an ethylene-inducible, GCC box DNA-binding protein interacts with an ocs element binding protein. Proc. Natl. Acad. Sci. USA 94: 5961–5966.
Chatel, G., Monteil, G., Pre´, M., Memelink, J., Thiersault, M., Saint-Pierre, B., Doireau, P. and Gantet, P. 2003. CrMYC1, a Catharanthus roseus elicitor-and jasmonate-responsive bHLH transcription factor that binds the G-box element of the strictosidine synthase gene promoter. J. Exp. Bot. 54: 2587–2588.
Chattopadhyay, M.K. and Ghosh, B. 1998. Molecular analysis of polyamine biosynthesis in higher plants. Curr. Sci. 74: 517–522.
Cheong, Y.H., Yoo, C.M., Park, J.M., Ryu, G.R., Goekjian, V.H., Nagao, R.T., Key, J.L., Cho, M.J. and Hong, J.C. 1998. STF1 is a novel TGACG-binding factor with zinc-finger motif and a bZIP domain which heterodimerizes with GBF proteins. Plant J. 15: 199–209.
Chou, W.M. and Kutchan, T.M. 1998. Enzymatic oxidations in the biosynthesis of complex alkaloids. Plant J. 15: 289–300.
De Luca, V. and St. Pierre, B. 2000. The cell and developmental biology of alkaloid biosynthesis. Trends Plant Sci. 5: 168–173.
Eilbert, U. 1998. Induction of alkaloid biosynthesis and accumulation in plants and in vitro cultures in response to elicitation. In: M.F. Roberts and M. Wink (Eds.) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press, New York, pp. 219–262.
Facchini, P.J. 2001. Alkaloid biosynthesis in plants: biochemistry, cell biology, molecular regulation and metabolic engineering applications. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 29–66.
van der Fits, L. and Memelink, J. 2000. ORCA3, a jasmonateresponsive transcriptional regulator of plant primary and secondary metabolism. Science 5477: 295–297.
van der Fits, L. and Memelink, J. 2001. The jasmonateinducible AP2/ERF-domain transcription factor ORCA3 activates gene expression via interaction with a jasmonateresponsive promoter element. Plant J. 25: 43–53.
Godoy, A.V., Zanetti, M.E., San Segundo, B. and Casalongue´, C. 2001. Identification of a putative Solanum tuberosum transcriptional coactivator up-regulated in potato tubers by Fusarium solani f. sp. eumartii infection and wounding. Physiol. Plant 112: 217–222.
Hansen, H. and Grossmann, K. 2000. Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol. 124:1437–1448.
Hashimoto, T. and Yamada, Y. 1994. Alkaloid biogenesis: molecular aspects. Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 257–285.
He, Y. and Gan, S. 2001. Identical promoter elements are involved in the regulation of the OPR1 gene by senescence and jasmonic acid in Arabidopsis. Plant Mol. Biol. 47:595–605.
Hehl, R. and Windgender, E. 2001. Database-assisted promoter analysis. Trends Plant Sci. 6: 251–255.
Heim, M.A., Jakoby, M., Werber, M., Martin, C., Weisshaar, B. and Bailey, P.C. 2003. The basic helix-loop-helix transcription factor family in plants: a genome-wide study of protein structure and functional diversity. Mol. Biol. Evol. 20: 735–747.
Hibi, N., Fujita, T., Hatano, M., Hashimoto, T. and Yamada, Y. 1992. Putrescine N-methyltransferase in cultured roots of Hyoscyamus albus. Plant Physiol. 100: 826–835.
Hibi, N., Higaahiguchi, S., Hashimoto, T. and Yamada, Y. 1994. Gene expression in tobacco low-nicotine mutants. Plant Cell 6: 723–735.
Imanishi, S., Hashizume, K., Nakakita, M., Kojima, H., Matsubayashi, Y., Hashimoto, T., Sakagami, Y., Yamada, Y. and Nakamura, K. 1998a. Differential induction by methyl jasmonate of genes encoding ornithine decarboxylase and other enzymes involved in nicotine biosynthesis in tobacco cell cultures. Plant Mol. Biol. 38: 1101–1111.
Imanishi, S., Hashizume, K., Kojima, H., Ichihara, A. and Nakamura, K. 1998b. An mRNA of tobacco cell, which is rapidly inducible by methyl jasmonate in the presence of cycloheximide, codes for a putative glycosyltransferase. Plant Cell Physiol. 39: 202–211.
Imanishi, S., Nakakita, M., Yamashita, K., Furuta, A., Utsuno, K., Muramoto, N., Kojima, H. and Nakamura, K. 2000. Aspirin and salicylic acid do not inhibit methyl jasmonate-inducible expression of a gene for ornithine decarboxylase in tobacco BY-2 cells. Biosci. Biotechnol. Biochem. 64:125–133.
Ishige, F., Takaichi, M., Foster, R., Chua, N-H. and Oeda, K. 1999. A G-box motif (GCCACGTGCC) tetramer confers high-level constitutive expression in dicot and monocot plants. Plant J. 18: 443–448.
Ishikawa, A., Yoshihara, T. and Nakamura, K. 1994. Jasmonate-inducible expression of a cathepsin D inhibitor-GUS gene fusion in tobacco cells. Plant Mol. Biol. 26: 403–414.
Jefferson, R.A., Kavanagh, T. A. and Bevan, M. W. 1987. GUS fusions: beta-glucuronidase as a sensitive and versatile gene marker in higher plants. EMBO J. 6: 3901–3907.
Kim, S.-R., Choi, J.-L, Costa, M.A. and An, G. 1992. Identification of G-box sequence as an essential element for methyl jasmonate response of potato proteinase inhibitor II promoter. Plant Physiol. 99: 627–631.
Kim, S.-R., Kim, Y. and An, G. 1993. Identification of methyl jasmonate and salicylic acid response elements from the nopaline synthase (nos) promoter. Plant Physiol. 103: 97–103.
Kutchan, T.M. 1998. Molecular genetics of plant alkaloid biosynthesis. In: G.A. Cordell (Ed.) The Alkaloids, Chemistry and Biology. Academic Press, San Diego, CA. pp. 295–304.
Malmberg, R.L., Watson, M.B., Galloway, G.L. and Yu, W. 1998. Molecular genetic analysis of plant polyamines. Critical Rev. Plant Sci. 17: 199–224.
Mason, H.S., De Wald, D. B. and Muller, J. E. 1993. Identi-fication of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell 5: 241–251.
Matsushita, Y., Miyakawa, O., Deguchi, M., Nishiguchi, M. and Nyunoya, H. 2002. Cloning of a tobacco cDNA coding for a putative transcriptional coactivator MBF1 that interacts with the tomato mosaic virus movement protein. J. Exp. Bot. 53: 1531–1532.
Legg, P. D. and Collins, G.B. 1971. Inheritance of percent total alkaloids in Nicotiana tabacum L. II. Genetic effects of two loci in Burley 21 · LA Burley 21 populations. Can. J. Gen. Cytol. 13: 287–291.
Legg, P.D., Chaplin, J.F. and Collins, G.B. 1969. Inheritance of percent total alkaloids in Nicotiana tabacum L. Populations derived from crosses of low alkaloid lines with burley and flue-cured varieties. J. Hered. 60: 213–217.
Memelink, J., Verpoorte, R. and Kijne, J. W. 2001. ORCAnization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci. 6: 212–219.
Menke, F.L.H., Champion, A., Kijne, J.W. and Memelink, J. 1999. A novel jasmonate-and elicitor-responsive element in the periwinkle secondary metabolite biosynthetic gene Str interacts with a jasmonate-and elicitor-inducible AP2-domain transcription factor, ORCA2. EMBO J.18: 4455–4463.
Menkens, A.E., Schindler, U. and Cashmore, A.R. 1995. The G-box: a ubiquitous regulatory element in plants bound by the GBF family of bZIP proteins. Trends Biochem. Sci. 20: 506–510.
Mizusaki, S., Tanebe, Y., Noguchi, M. and Tamaki, E. 1973. Changes in activities of ornithine decarboxylase, putrescine N-methyltransferase and N-methylputrescine oxidase in tobacco roots in relation to nicotine biosynthesis. Plant Cell Physiol. 14: 103–110.
Murashige, T. and Skoog, F. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant 15: 473–479.
Ohme-Takagi, M., Suzuki, K. and Shinshi, H. 2000. Regulation of ethylene-induced transcription of defense genes. Plant Cell Physiol. 41: 1187–1192.
Ohnmeiss, T.E., McCloud, E.S., Lynds, G.Y. and Baldwin, F.T. 1997. Within-plant relationships among wounding, jasmonic acid, and nicotine: implications for defense in Nicotiana sylvestris. New Phytologist 137: 441–452.
Ouwerkerk, P.B.F. and Memelink, J. 1999. A G-box element from the Catharanthus roseus strictosidine synthase (Str) gene promoter confers seed-specific expression in transgenic tobacco plants. Mol. Gen. Genet. 261: 635–643.
Park, J. M., Park, C.J., Lee, S.B., Ham, B.K., Shin, R. and Paek, K.H. 2001. Overexpression of the tobacco Tsi1 gene encoding an EREBP/ AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13: 1035–1046.
Pasquali, G., Erven, A.S.W., Ouwerkerk, P.B.F., Menke, F. L.H. and Memelink, J. 1999. The promoter of the strictosidine synthase gene from periwinkle confers elicitorinducible expression in transgenic tobacco and binds nuclear factors GT-l and GBF. Plant Mol. Biol. 39: 1299–1310.
Pre´, M., Sibe´ ril, Y., Memelink, J., Champion, A., Doireau, P. and Gantet, P. 2000. Isolation by the yeast one-hybrid system of cDNAs encoding transcription factors that bind to the G-box element of the strictosidine synthase gene promoter from Catharanthus roseus. Int. J. Bio-Chrom. 5: 229–244.
Riechers, D.E. and Timko, M.P. 1999. Structure and expression of the gene family encoding putrescine N-methyltransferase in Nicotiana tobacum: new clues to the evolutionary origin of cultivated tobacco. Plant Mol. Biol. 41: 387–401.
Rouster, J., Leah, R., Mundy, J. and Cameron-Mills, V. 1997. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase1 gene expressed in barley grain. Plant J. 11: 513–523.
Sachan, N. and Falcone, D.L. 2002. Wound-induced gene expression of putrescine N-methyltransferase in leaves of Nicotiana tabacum. Phytochemistry 7: 797-805.
Saito, K. and Murakoshi, L. 1998. Genes in alkaloid metabolism. In: M.F. Roberts and M. Wink (Eds.) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press, New York, pp. 147–157.
Sato, F., Hashimoto, T., Hachiya, A.T., Choi, K., Morishige, T., Fujimoto, H. and Yamada, Y. 2001. Metabolic engineering of plant alkaloid biosynthesis. Proc. Natl. Acad. Sci. USA 98: 367–372.
Saunders, J.W. and Bush, L.P. 1979. Nicotine biosynthesis enzyme activities in Nicotiana tabacum L. genotypes with different alkaloid levels. Plant Physiol. 64: 236–240.
Schindler, U., Beckmann, H. and Cashmore, A.R. 1992a. TGA1 and G-box binding factors: two distinct classes of Arabidopsis leucine zipper proteins compete for the G-boxlike element TGACGTGG. Plant Cell 4: 1309–1319.
Schindler, U., Menkens, A.E., Beckmann, H., Ecker, J.R. and Cashmore, A.R. 1992b. Heterodimerization between lightregulated and ubiquitously expressed Arabidopsis GBF bZIP proteins. EMBO J. 11: 1261–1273.
Shoji, T., Yamada, Y. and Hashimoto, T. 2000a. Jasmonate induction of putrescine N-methyltransferase genes in the root of Nicotiana sylvestris. Plant Cell Physiol. 41: 831–839.
Shoji, T., Nakajima, K. and Hashimoto, T. 2000b. Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol. 41: 1072–1076.
Sibe´ ril, Y., Doireau, P. and Gantet, P. 2001. Plant bZIP G-box binding factors: molecular structure and activation mechanisms. Eur J. Biochem. 268: 5655–5666.
Sinclair, S.J., Murphy, K.J., Birch, C.D. and Hamil, J.D. 2000. Molecular characterization of quinolinate phosphoribosyltransferase (QPRTase) in Nicotiana. Plant Mol. Biol. 44: 603–617.
Skinner, J.S. and Timko, M.P. 1999. Differential expression of genes encoding the light-dependent and light-independent enzymes for protochlorophyllide reduction during development in loblolly pine. Plant Mol. Biol. 39: 577–592.
Suzuki, K., Yamada, Y. and Hashimoto, T. 1999. Expression of Atropa belladonna putrescine N-methyltransferase gene in root pericycle. Plant Cell Physiol. 40: 289–297.
Tsuda, K., Tsuji, T., Hirose, S. and Yamazaki, K. 2004. Three Arabidopsis MBF1 homologs with distinct expression pro-files play roles as transcriptional co-activators. Plant Cell Physiol. 45: 225–231.
Voelckel, C., Kriiger, T., Gase1, K., Heidrich, N., van Dam, N.M., Winz, R. and Baldwin, I.T. 2001. Anti-sense expression of putrescine N-methyltransferase confirms defensive role of nicotine in Nicotiana sylvestris against Manduca sexta. Chemoecology 11: 121–126.
Waterman, P.M. 1998. Chemical taxonomy of alkaloids. In: M.F. Roberts and M. Wink (Eds.) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press, New York, pp. 87–107.
Wink, M., 1998. Modes of action of alkaloids. In: M.F. Roberts and M. Wink (Eds.) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Plenum Press, New York. pp. 301–326.
Winz, R.A. and Baldwin, I.T. 2001. Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. IV. Insect-induced ethylene reduces jasmonate-induced nicotine accumulation by regulating putrescine N-methyltransferase transcripts. Plant Physiol. 125: 2189–2202.
Xiang, C., Miao, Z. and Lam, E. 1996. Coordinated activation of as-l-type elements and a tobacco glutathione S-transferase gene by auxins, salicylic acid, methyl-jasmonate and hydrogen peroxide. Plant Mol. Biol. 32: 415–426.
Zegzouti, H., Jones, B., Frasse, P., Marty, C., Maitre, B., Latche´, A., Pech, J.C. and Bouzayen, M. 1999. Ethyleneregulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 18: 589–600.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Xu, B., Timko, M. Methyl jasmonate induced expression of the tobacco putrescine N-methyltransferase genes requires both G-box and GCC-motif elements. Plant Mol Biol 55, 743–761 (2004). https://doi.org/10.1007/s11103-004-1962-8
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-1962-8