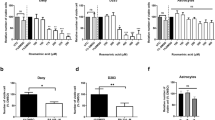
Abstract
Malignant gliomas are a highly aggressive type of brain tumor with extremely poor prognosis. These tumors are highly invasive and are often surgically incurable and resistant to chemotherapeutics and radiotherapy. Thus, novel therapies that target pathways involved in growth and survival of the tumor cells are required for the treatment of this class of brain tumors. Previous studies revealed that epidermal growth factor receptor and extracellular-signal-regulated kinases (ERKs), which are involved in the induction of cell proliferation, are activated in the most aggressive type of glioma, i.e. glioblastoma multiforme (GBM). In fact, GBMs with increased levels of ERK activity exhibit a more aggressive phenotype than the others with moderate ERK activity, pointing to the importance of ERK and its kinase activity in the development and progression of these tumors. In this study, we have evaluated the effect of p38SJ, a novel member of the DING family of proteins, derived from Hypericum perforatum calluses, on the growth of malignant glioma cell lines, T98G and U-87MG by focusing on cell cycle and signaling pathways controlled by phosphorylation of various regulatory proteins including ERK. p38SJ, which exhibits profound phosphatase activity, shows the capacity to affect the phosphorylation status of several important kinases modulating signaling pathways, and cell growth and proliferation. Our results demonstrate that p38SJ reduces glioma cell viability and arrests cell cycle progression at G0/G1. The observed growth inhibitory effect of p38SJ is likely mediated by the downregulation of several cell cycle gatekeeper proteins, including cyclin E, Cdc2, and E2F-1. These results suggest that p38SJ may serve as a potential candidate for development of a therapeutic agent for the direct treatment of malignant gliomas and/or as a potential radiosensitizer.






Similar content being viewed by others
References
Karioti A, Bilia AR (2010) Hypericins as potential leads for new therapeutics. Int J Mol Sci 11:562–594
Suzuki O, Katsumata Y, Oya M et al (1984) Inhibition of monoamine oxidase by hypericin. Planta Med 50:272–274
Lavie G, Mandel M, Hazan S et al (2005) Anti-angiogenic activities of hypericin in vivo: potential for ophthalmologic applications. Angiogenesis 8:35–42
Davids LM, Kleemann B, Kacerovskà D et al (2008) Hypericin phototoxicity induces different modes of cell death in melanoma and human skin cells. J Photochem Photobiol B 91:67–76
Thomas C, Pardini RS (1992) Oxygen dependence of hypericin-induced phototoxicity to EMT6 mouse mammary carcinoma cells. Photochem Photobiol 55:831–837
Agostinis P, Vantieghem A, Merlevede W et al (2002) Hypericin in cancer treatment: more light on the way. Int J Biochem Cell Biol 34:221–241
Ritz R, Wein HT, Dietz K, Schenk M, Roser F, Tatagiba M, Strauss WS (2007) Photodynamic therapy of malignant glioma with hypericin: comprehensive in vitro study in human glioblastoma cell lines. Int J Oncol 30(3):659–667
Schneider-Yin X, Kurmanaviciene A, Roth M et al (2009) Hypericin and 5-aminolevulinic acid-induced protoporphyrin IX induce enhanced phototoxicity in human endometrial cancer cells with non-coherent white light. Photodiagnosis Photodyn Ther 6:12–18
Kleban J, Szilardiova B, Mikes J et al (2006) Pre-treatment of HT-29 cells with 5-LOX inhibitor (MK-886) induces changes in cell cycle and increases apoptosis after photodynamic therapy with hypericin. J Photochem Photobiol B 84:79–88
Duo H, Olivo M, Mahendran R et al (2004) Modulation of matrix metalloproteinase-1 in nasopharyngeal cancer cells by photoactivation of hypericin. Int J Oncol 24:657–662
Miccoli L, Beurdeley-Thomas A, De PG et al (1998) Light-induced photoactivation of hypericin affects the energy metabolism of human glioma cells by inhibiting hexokinase bound to mitochondria. Cancer Res 58:5777–5786
Berlanda J, Kiesslich T, Oberdanner CB et al (2006) Characterization of apoptosis induced by photodynamic treatment with hypericin in A431 human epidermoid carcinoma cells. J Environ Pathol Toxicol Oncol 25:173–188
Assefa Z, Vantieghem A, Declercq W et al (1999) The activation of the c-Jun N-terminal kinase and p38 mitogen-activated protein kinase signaling pathways protects HeLa cells from apoptosis following photodynamic therapy with hypericin. J Biol Chem 274:8788–8796
Buytaert E, Matroule JY, Durinck S et al (2008) Molecular effectors and modulators of hypericin-mediated cell death in bladder cancer cells. Oncogene 27:1916–1929
Darbinian N, Czernik M, Darbinyan A, Elias M, Chabriere E, Bonasu S, Khalili K, Amini S (2009) Evidence for phosphatase activity of p27SJ and its impact on the cell cycle. J Cell Biochem 107(3):400–407
Darbinian-Sarkissian N, Darbinyan A, Otte J et al (2006) p27(SJ), a novel protein in St John’s Wort, that suppresses expression of HIV-1 genome. Gene Ther 13:288–295
Darbinian N, Gomberg R, Mullen L et al (2011) Suppression of HIV-1 transcriptional elongation by a DING phophatase. J Cell Biochem 112:225–232
Diemer H, Elias M, Renault F et al (2008) Tandem use of X-ray crystallography and mass spectrometry to obtain ab initio the complete and exact amino acids sequence of HPBP, a human 38-kDa apolipoprotein. Proteins 71:1708–1720
Riah O, Dousset JC, Bofill-Cardona E et al (2000) Isolation and microsequencing of a novel cotinine receptor. Cell Mol Neurobiol 20:653–664
Berna A, Bernier F, Scott K et al (2002) Ring up the curtain on DING proteins. FEBS Lett 524:6–10
Berna A, Bernier F, Chabriere E et al (2008) DING proteins; novel members of a prokaryotic phosphate-binding protein superfamily which extends into the eukaryotic kingdom. Int J Biochem Cell Biol 40:170–175
Belenky M, Prasain J, Kim H, Barnes S (2003) DING, a genistein target in human breast cancer: a protein without a gene. J Nutr 133(Suppl 7):2497S–2501S
Scott K, Wu L (2005) Functional properties of a recombinant bacterial DING protein: comparison with a homologous human protein. Biochim Biophys Acta 1744:234–244
Ahn S, Moniot S, Elias M et al (2007) Structure-function relationships in a bacterial DING protein. FEBS Lett 581:3455–3460
Moniot S, Elias M, Kim D et al (2007) Crystallization, diffraction data collection and preliminary crystallographic analysis of DING protein from Pseudomonas fluorescens. Acta Crystallograph Sect F 63(Pt 7):590–592
Chen Z, Franco CF, Baptista RP et al (2007) Purification and identification of cutinases from Colletotrichum kahawae and Colletotrichum gloeosporioides. Appl Microbiol Biotechnol 73:1306–1313
Pantazaki AA, Tsolkas GP, Kyriakidis DA (2007) A DING phosphatase in Thermus thermophilus. Amino Acids 34:437–448
Perera T, Berna A, Scott K, Lemaitre-Guillier C et al (2008) Proteins related to St. John’s Wort p27SJ, a suppressor of HIV-1 expression, are ubiquitous in plants. Phytochemistry 69:865–872
Lesner A, Li Y, Nitkiewicz J, Li G, Kartvelishvili A, Kartvelishvili M, Simm M (2005) A soluble factor secreted by an HIV-1 resistant cell line blocks transcription through inactivating the DNA-binding capacity of the NF-κB p65/p50 dimer. J Immunol 175:2548–2554
Amini S, Merabova N, Khalili K, Darbinian N (2009) p38SJ, a novel DINGG protein protects neuronal cells from alcohol induced injury and death. J Cell Physiol 221(3):499–504
Tsai LH, Harlow E, Meyerson M (1991) Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature 353:174–177
Meyerson M, Enders GH, Wu CL et al (1992) A family of human cdc2-related protein kinases. EMBO J 11:2909–2917
Neuman E, Sellers WR, McNeil JA et al (1996) Structure and partial genomic sequence of the human E2F1 gene. Gene 173:163–169
Morgan, David L (2007) The cell cycle: principles of control. New Science Press, London, pp 30–31
Amini S, Clavo A, Nadraga Y et al (2002) Interplay between cdk9 and NF-kappaB factors determines the level of HIV-1 gene transcription in astrocytic cells. Oncogene 21:5797–5803
Romano G, Giordano A (2008) Role of the cylcin-dependent kinase 9-related pathway in mammalian gene expression and human disease. Cell Cycle 7:3664–3668
Pirngruber J, Johnsen SA (2010) Induced G1 cell-cycle arrest controls replication-dependent histone mRNA 3′ end processing through p21, NPAT and CDK9. Oncogene 29:2853–2963
Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF (2000) Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog 28:102–110
Yoshida J, Ishibashi T, Nishio M (2007) G1 cell cycle arrest by amlodipine, a dihydropyridine Ca2+ channel blocker, in human epidermoid carcinoma A431 cells. Biochem Pharmacol 73(7):943–953
Brady G, Boggan L, Bowie A, O’Neill LA (2005) Schlafen-1 causes a cell cycle arrest by inhibiting induction of cyclin D1. J Biol Chem 280(35):30723–30734
Sonoda Y, Ozawa T, Aldape KD et al (2001) Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyt model of glioma. Cancer Res 61:6674–6678
Mure H, Matsuzaki K, Kitazato KT et al (2010) Akt2 and Akt3 play a pivotal role in malignant gliomas. Neuro Onc 12:221–232
Huang L, Li B, Li W et al (2009) ATP-sensitive potassium channels control glioma cells proliferation by regulating ERK activity. Carcinogenesis 30:737–744
Zhang L, Ma Y, Zhang J, Cheng J, Du J (2005) A new cellular signaling mechanism for angiotensin II activation of NF-kappaB: an IkappaB-independent, RSK-mediated phosphorylation of p65. Arterioscler Thromb Vasc Biol 25(6):1148–1153 Epub 2005 Mar 31
Santi SA, Lee H (2011) Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One 6(1):e14614
Wang M, Atayar C, Rosati S, Bosga-Bouwer A, Kluin P, Visser L (2009) JNK is constitutively active in mantle cell lymphoma: cell cycle deregulation and polyploidy by JNK inhibitor SP600125. J Pathol 218(1):95–103
Gangadhar NM, Firestein SJ, Stockwell BR (2008) A novel role for jun N-terminal kinase signaling in olfactory sensory neuronal death. Mol Cell Neurosci 38(4):518–525
Acknowledgments
The authors wish to thank past and present members of the Department of Neuroscience and Center for Neurovirology for sharing reagents and ideas, and for their continued support. We thank Armine Darbinyan, M.D. for contributing to various aspects of this work, and Samantha Garcia for her assistance with the quantitative analysis of the cell viability assay data. We are grateful to Dr. G. Tuszynski and Dr. M. K. White for critical reading of this manuscript. This work was supported by Tumor Research Grant from Synthes North America to the Temple University School of Medicine Neurosurgery Department, funded, in part, under a grant with the Pennsylvania Department of Health to N. Darbinian, and NIH grants to K. Khalili and S. Amini.
Conflicts of interest
No potential conflicts of interest were disclosed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Markus J. Bookland and Nune Darbinian contributed equally to this study.
Rights and permissions
About this article
Cite this article
Bookland, M.J., Darbinian, N., Weaver, M. et al. Growth inhibition of malignant glioblastoma by DING protein. J Neurooncol 107, 247–256 (2012). https://doi.org/10.1007/s11060-011-0743-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-011-0743-x