Abstract
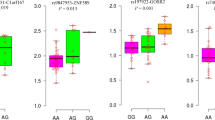
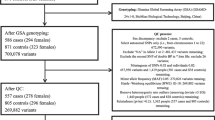
Genome-wide association studies have identified a large number of genetic loci for blood pressure in European populations. The associations in other populations are needed to determine. The purpose of this study was to examine the associations between the single nucleotide polymorphisms (SNPs) identified in European populations and hypertension in the Chinese Han population, and highlight the potential roles. Seven tag-SNPs were genotyped in 857 hypertension cases and 927 controls to test the associations. The intronic SNP rs10224002 (PRKAG2) which could affect DNase and regulatory motif was associated with hypertension (P = 0.024). This SNP was also found to be associated with coronary artery disease and stroke. We searched for potential functional variants by bioinformatics analysis and found various kinds of variants such as missense mutations, phosphorylation-related SNPs and SNPs that have regulatory potentials in the blood pressure loci. We performed expression quantitative trait locus (eQTL) and differential expression analyses for the identified variants and genes. eQTL analysis found that rs10224002 was associated with PRKAG2 gene expression in peripheral blood (P = 0.0016). PRKAG2 was differentially expressed between hypertension cases and controls (P = 0.0133), coronary artery disease cases and controls (P = 0.02112) and stroke cases and controls (P = 0.0059). Our study demonstrated that SNPs rs10224002 may be associated with hypertension in the Chinese Han population and PRKAG2 may play a role in the etiology of hypertension and cardiovascular diseases.


Similar content being viewed by others
References
Poulter NR, Prabhakaran D, Caulfield M (2015) Hypertension. Lancet 386(9995):801–812. https://doi.org/10.1016/S0140-6736(14)61468-9
Forouzanfar MH, Liu P, Roth GA et al (2017) Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 317(2):165–182. https://doi.org/10.1001/jama.2016.19043
Lewington S, Lacey B, Clarke R et al (2016) The burden of hypertension and associated risk for cardiovascular mortality in China. JAMA Intern Med 176(4):524–532. https://doi.org/10.1001/jamainternmed.2016.0190
Kupper N, Willemsen G, Riese H et al (2005) Heritability of daytime ambulatory blood pressure in an extended twin design. Hypertension 45(1):80–85. https://doi.org/10.1161/01.HYP.0000149952.84391.54
Warren HR, Evangelou E, Cabrera CP et al (2017) Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat Genet 49(3):403–415. https://doi.org/10.1038/ng.3768
Tragante V, Barnes MR, Ganesh SK et al (2014) Gene-centric meta-analysis in 87,736 individuals of European ancestry identifies multiple blood-pressure-related loci. Am J Hum Genet 94(3):349–360. https://doi.org/10.1016/j.ajhg.2013.12.016
Giri A, Hellwege JN, Keaton JM et al (2019) Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat Genet 51(1):51–62. https://doi.org/10.1038/s41588-018-0303-9
Ward LD, Kellis M (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucl Acids Res 40(D1):D930–D934. https://doi.org/10.1093/nar/gkr917
Korkor MT, Meng FB, Xing SY et al (2011) Microarray analysis of differential gene expression profile in peripheral blood cells of patients with human essential hypertension. Int J Med Sci 8(2):168–179. https://doi.org/10.7150/ijms.8.168
Krug T, Gabriel JP, Taipa R et al (2012) TTC7B emerges as a novel risk factor for ischemic stroke through the convergence of several genome-wide approaches. J Cereb Blood Flow Metab 32(6):1061–1072. https://doi.org/10.1038/jcbfm.2012.24
Zheng Y, Nie P, Peng D et al (2018) m6AVar: a database of functional variants involved in m6A modification. Nucl Acids Res 46(D1):D139–D145. https://doi.org/10.1093/nar/gkx895
Ren J, Jiang C, Gao X et al (2010) PhosSNP for systematic analysis of genetic polymorphisms that influence protein phosphorylation. Mol Cell Proteom 9(4):623–634. https://doi.org/10.1074/mcp.M900273-MCP200
Bhattacharya A, Ziebarth JD, Cui Y (2014) PolymiRTS Database 3.0: linking polymorphisms in microRNAs and their target sites with human diseases and biological pathways. Nucl Acids Res 42(D1):D86–D91. https://doi.org/10.1093/nar/gkt1028
Guo L, Wang J (2018) rSNPBase 3.0: an updated database of SNP-related regulatory elements, element-gene pairs and SNP-based gene regulatory networks. Nucl Acids Res 46(D1):D1111–D1116. https://doi.org/10.1093/nar/gkx1101
Westra HJ, Peters MJ, Esko T et al (2013) Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet 45(10):1238–1243. https://doi.org/10.1038/ng.2756
Kheradpour P, Kellis M (2014) Systematic discovery and characterization of regulatory motifs in ENCODE TF binding experiments. Nucl Acids Res 42(5):2976–2987. https://doi.org/10.1093/nar/gkt1249
Ryu GM, Song P, Kim KW et al (2009) Genome-wide analysis to predict protein sequence variations that change phosphorylation sites or their corresponding kinases. Nucl Acids Res 37(4):1297–1307. https://doi.org/10.1093/nar/gkn1008
Kelly TN, Takeuchi F, Tabara Y et al (2013) Genome-wide association study meta-analysis reveals transethnic replication of mean arterial and pulse pressure loci. Hypertension 62(5):853–859. https://doi.org/10.1161/hypertensionaha.113.01148
Lu X, Wang L, Lin X et al (2015) Genome-wide association study in Chinese identifies novel loci for blood pressure and hypertension. Hum Mol Genet 24(3):865–874. https://doi.org/10.1093/hmg/ddu478
Ganesh SK, Zakai NA, van Rooij FJ et al (2009) Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nat Genet 41(11):1191–1198. https://doi.org/10.1038/ng.466
Zakai NA, Katz R, Hirsch C et al (2005) A prospective study of anemia status, hemoglobin concentration, and mortality in an elderly cohort: the cardiovascular health study. Arch Intern Med 165(19):2214–2220. https://doi.org/10.1001/archinte.165.19.2214
Wannamethee G, Shaper AG (1994) Haematocrit: relationships with blood lipids, blood pressure and other cardiovascular risk factors. Thromb Haemost 72(1):58–64
Gagnon DR, Zhang TJ, Brand FN, Kannel WB (1994) Hematocrit and the risk of cardiovascular disease–the Framingham study: a 34-year follow-up. Am Heart J 127(3):674–682. https://doi.org/10.1016/0002-8703(94)90679-3
Elwood PC, Waters WE, Benjamin IT, Sweetnam PM (1974) Mortality and anaemia in women. Lancet 1(7863):891–894. https://doi.org/10.1016/S0140-6736(74)90346-8
Sarnak MJ, Tighiouart H, Manjunath G et al (2002) Anemia as a risk factor for cardiovascular disease in the atherosclerosis risk in Communities (ARIC) study. J Am Coll Cardiol 40(1):27–33. https://doi.org/10.1016/S0735-1097(02)01938-1
Nikpay M, Goel A, Won HH et al (2015) A comprehensive 1000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet 47(10):1121–1130. https://doi.org/10.1038/ng.3396
Malik R, Chauhan G, Traylor M et al (2018) Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet 50(4):524–537. https://doi.org/10.1038/s41588-018-0058-3
Eslam M, McLeod D, Kelaeng KS et al (2017) IFN-lambda3, not IFN-lambda4, likely mediates IFNL3-IFNL4 haplotype-dependent hepatic inflammation and fibrosis. Nat Genet 49(5):795–800. https://doi.org/10.1038/ng.3836
Acknowledgements
The study was supported by the Key Research Project (Social Development Plan) of Jiangsu Province (Grant No. BE2016667), Natural Science Foundation of China (Grant Nos. 81773508, 81302499 and 81320108026), Project funded by China Postdoctoral Science Foundation (Grant Nos. 2014T70547 and 2013M530269), the Startup Fund from Soochow University (Grant Nos. Q413900313, Q413900412), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical approval
This study was approved by the ethics committee at Soochow University in China.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mo, X., Zhang, H., Zhou, Z. et al. SNPs rs10224002 in PRKAG2 may disturb gene expression and consequently affect hypertension. Mol Biol Rep 46, 1617–1624 (2019). https://doi.org/10.1007/s11033-019-04610-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-019-04610-3