Abstract
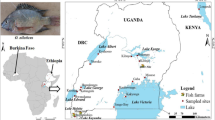
The Cichlid fish Sarotherodon melanotheron is typically found in West and Central African estuaries and lagoons. It represents a good candidate for promoting tilapia farming in brackish waters. Understanding the genetic diversity in its populations from the hydrographical basins of Southern Benin is primordial before designing selective breeding programs. For this purpose, 202 samples collected from four rivers of Southern Benin and were genotyped using 15 polymorphic microsatellite DNA markers. Each river was split up into three sampling sites. We found significant global linkage disequilibrium across the genome of natural populations of this tilapia species overall the loci. However, when the loci that display aberrant Wright's (FIS and FST) were removed from the data, a linkage disequilibrium was detected for the remaining 11 loci and became compatible with the null hypothesis. Null alleles explained at least 20.58% of FIS variation. We found a significant isolation by distance across subsamples. Effective population size averaged 210 individuals, with a range from 36 to 517 individuals. Assuming that 79% of heterozygote deficits are explained by sib mating lead to a rough estimate of rsm = 0.4 of mating rate between full sibs within S. melanotheron subpopulations. The fish size correlated positively and significantly with the observed FIS (r = 0.58; p value = 0.04806). Reproduction system (endogamy) in S. melanotheron could explain the strong heterozygote deficit observed. Our results provide technical guidance for efficient management of this tilapia species’ genetic resources for breeding programs in fresh and brackish waters.











Similar content being viewed by others
References
Lalèyè P, Chikou A, Philippart JC, Teugels G, Vandewalle P (2004) Etude de la diversité ichtyologique du bassin du fleuve Ouémé au Bénin (Afrique de l’Ouest). Cybium 28(1):329–339
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev Camb Philos Soc 8(2):163–182
Ndiaye PG, Bakanova B, Bihibindi A, Gaye AT, Gueye C, Sambou B, Sene MM (2010) Pêche et changements climatiques en Afrique de l’Ouest: État des lieux. Dakar. Réseau sur les Politiques de Pêche en Afrique de l’Ouest (REPAO), Sénégal
Payne AI, Cowan V (1998) Review of stock enhancement in the floodplains of Bangladesh. FAO, Rome, p 6
Gustafson KA (1988) Management briefs: approximating confidence intervals for indices of fish population size structure. North Am J Fish Manag 8(1):139–141
Birkeland C, Dayton PK (2005) The importance in fishery management of leaving the big ones. Trends Ecol Evol 20(7):356–358
Abban EK, Casal CMV, Dugan P, Falk TM (2004) Biodiversity, management and utilization of West African fishes. In: WorldFish Center Conference Proceedings. WorldFish Center, Penang
Nguyen T, Hurwood D, Mather P, Na-Nakorn U, Komonrat W, Bartley D (2006) Manual on application of molecular tools in aquaculture and inland. Fisheries management—Part 2: laboratory protocols and data analysis. FAO, Rome
Nikolic N, Fève K, Chevalet C, Høyheim B, Riquet J (2009) A set of 37 microsatellite DNA markers for genetic diversity and structure analysis of Atlantic Salmon Salmo salar Populations. J Fish Biol 74(2):458–466
Dudgeon CL, Blower DC, Broderick D, Giles JL, Holmes BJ, Kashiwagi T, Krück NC, Morgan JT, Tillett BJ, Ovenden JR (2012) A review of the application of molecular genetics for fisheries management and conservation of sharks and rays. J Fish Biol 80(5):1789–1843
Ollivier L, Chevalet C, Foullet JL (2000) Utilisation des marqueurs pour la caractérisation des ressources génétiques. INRA Prod Anim HS:247–252
Ambali AJD, Malekano LB (2004) Genetic improvement with specific reference to tilapia genetic resources in africa and their use in aquaculture—potential benefits and risks. In: WorldFish Center Conference Proceedings. WorldFish Center, Penang, pp 11–15
Brinez B, Caraballo X, Salazar M (2011) Genetic diversity of six populations of red hybrid tilapia, using microsatellites genetic markers. Rev MVZ Córdoba 16(2):2491–2498
Bezault E, Balaresque P, Toguyeni A, Fermon Y, Araki H, Baroiller JF, Rognon X (2011) Spatial and temporal variation in population genetic structure of wild Nile Tilapia (Oreochromis niloticus) across Africa. BMC Genet 12(1):1–16
Amoussou TO, Toguyeni A, Imorou Toko I, Chikou A, Bossou MA, Youssao Abdou Karim I (2016) Morphological diversity of wild populations of Sarotherodon melanotheron Rüppell, 1852 of Southern Benin. J Anim Sci Adv 6(11):1811–1830
Amoussou TO, Toguyeni A, Imorou Toko I, Chikou A, Youssao Abdou Karim I (2016) Biological and zootechnical characteristics of African tilapias Oreochromis niloticus (Linnaeus, 1758) and Sarotherodon melanotheron Rüppell, 1852: a review. Int J Biol Chem Sci 10(4):1869–1887
Pouyaud L, Agnèse JF (1994) Différenciation génétique des populations de Sarotherodon melanotheron, Rüppell, 1853. In: Atelier biodiversité et aquaculture en Afrique. ORSTOM, Abidjan, pp 66–73
Adépo-Gourène B, Pouyaud L, Teugels GG, Hanssens MM, Agnèse JF (1998) Morphological and genetic differentiation of west african populations of Sarotherodon melanotheron Rüppell, 1852 (Teleostei, Cichlidae). In: Agnèse J-F (ed) Genetics and aquaculture in Africa. ORSTOM, Paris, pp 189–198
Falk TM, Teugels GG, Abban EK (2000) Genetic characterization of West African populations of Sarotherodon melanotheron (Teleostei, Cichlidae). In: Abban EK, Casal CMV, Falk TM, Pullin RSV (eds) Biodiversity and sustainable use of fish in the coastal zone. International Center for Living Aquatic Resources Management, Penang, pp 8–11
Falk TM, Teugels GG, Abban EK, Villwock W, Renwrantz L (2003) Phylogeographic patterns in populations of the black-chinned Tilapia complex (Teleostei, Cichlidae) from coastal areas in West Africa: support for the refuge zone theory. Mol Phylog Evol 27(1):81–92
Yoboué AN, Adepo-Gourène BA, Séka D, Durand JD, Panfili J, Laë R (2012) Genetic diversity and adaptability of Sarotherodon melanotheron (Cichlidae) in coastal ecosystem. Ethol Ecol Evol 24(3):230–243
Usman BA, Agbebi OT, Bankole MO, Oguntade OR, Popoola MO (2013) Molecular characterisation of two Cichlids populations (Tilapia guineensis and Sarotherodon melanotheron) from different water bodies in Lagos State, Nigeria. Int J Biotechnol Mol Biol Res 4(5):71–77
Chistiakov DA, Hellemans B, Volckaert FAM (2006) Microsatellites and their genomic distribution, evolution, function and applications: a review with special reference to fish genetics. Aquaculture 255(1–4):1–29
Yoboue AN, Adepo-Gourene AB, Agnese JF, Laë R (2014) Diversité et structure génétique de Sarotherodon melanotheron (Pisces:Cichlidae) révélées par les microsatellites. Eur Sci J 10(33):299–311
Bezault E, Rognon X, Gharbi K, Baroiller JF, Chevassus B (2012) Microsatellites cross-species amplification across some African Cichlids. Int J Evol Biol. https://doi.org/10.1155/2012/870935
Coombs JA, Letcher BH, Nislow KH (2008) CREATE: a software to create input files from diploid genotypic data for 52 genetic software programs. Mol Ecol Resour 8(3):578–580
Goudet J (2003) FSTAT (Version 2.9.4), a program (for windows 95 and above) to estimate and test population genetics parameters. Department of Ecology & Evolution, Lausanne University, Switzerland, p 53
De Meeûs T, Guégan JF, Teriokhin AT (2009) MultiTest V.1.2, a program to binomially combine independent tests and performance comparison with other related methods on proportional data. BMC Bioinform 10:1–8
R-Development-Core-Team (2016) R: A language and environment for statistical computing. Version 3.3.2 (2016-10-31). R Foundation for Statistical Computing, Vienna
Benjamini Y, Yekutieli D (2001) The control of the false discovery rate in multiple testing under dependency. Ann Stat 29(4):1165–1188
De Meeûs T (2014) Statistical decision from k test series with particular focus on population genetics tools: a DIY notice. Inf Genet Evol 22:91–93
Wright S (1965) The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19(3):395–420
Nagylaki T (1998) Fixation indices in sudivided populations. Genetics 148(3):1325–1332
De Meeûs T (2012) Initiation à la génétique des populations naturelles: application aux parasites et à leurs vecteurs. IRD Editions, Marseilles
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38(6):1358–1370
Goudet J, Raymond M, De Meeus T, Rousset F (1996) Testing differentiation in diploid populations. Genetics 144(4):1933–1940
De Meeûs T (2018) Revisiting FIS, FST, Wahlund effects and null alleles. J Hered. https://doi.org/10.1093/jhered/esx106
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) Micro-Checker: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4(3):535–538
De Meeûs T, Humair PF, Grunau C, Delaye C, Renaud F (2004) Non-mendelian transmission of alleles at microsatellite loci: an example in Ixodes ricinus, the vector of lyme disease. Int J Parasitol 34(8):943–950
Fox J (2005) The R commander: a basic statistics graphical user interface to R. J Stat Softw 44(9):1–42
Fox J (2007) Extending the R commander by “plug-in” packages. R News 7:46–52
Chevillon C, Koffi BB, Barré N, Durand P, Arnathau C, De Meeûs T (2007) Direct and indirect inferences on parasite mating and gene transmission patterns. Pangamy in the Cattle tick Rhipicephalus (Boophilus) microplus. Inf Genet Evol 7(2):298–304
Nei M, Chesser RK (1983) Estimation of fixation indices and gene diversities. Ann Hum Genet 47:253–259
El Mousadik A, Petit RJ (1996) High level of genetic differentiation for allelic richness among populations of the argan tree Argania spinosa (L.) skeels endemic to Morocco. Theor Appl Genet 92(7):832–839
Meirmans PG, Hedrick PW (2011) Assessing population structure: FST and related measures’. Mol Ecol Resour 11(1):5–18
Rousset F (1997) Genetic differentiation and estimation of gene flow from F-statistics under isolation by distance. Genetics 145(4):1219–1228
Séré M, Thévenon S, Belem AMG, De Meeûs T (2017) Comparison of different genetic distances to test isolation by distance between populations. Heredity 119:55–63
Cavalli-Sforza LL, Edwards AWF (1967) Phylogenetic analysis: model and estimation procedures. Evolution 21(3):550–570
Chapuis MP, Estoup A (2007) Microsatellite null alleles and estimation of population differentiation. Mol Biol Evol 24(3):621–631
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for windows and linux. Mol Ecol Resour 8(1):103–106
Rousset F (2015) Genepop’4.4: a complete reimplementation of the Genepop software for Windows/Linux/Mac OS X. p 55
Do C, Waples RS, Peel D, Macbeth GM, Tillett BJ, Ovenden JR (2014) NeEstimator v2: re-implementation of software for the estimation of contemporary effective population size (Ne) from genetic data. Mol Ecol Resour 14(1):209–214
Peel D, Waples RS, Macbeth GM, Do C, Ovenden JR (2013) Accounting for missing data in the estimation of contemporary genetic effective population size (Ne). Mol Ecol Resour 13(2):243–253
Nomura T (2008) Estimation of effective number of breeders from molecular coancestry of single cohort sample. Evol Appl 1(3):462–474
Vitalis R, Couvet D (2001) Estimation of effective population size and migration rate from one- and two-locus identity measures. Genetics 157:911–925
Vitalis R, Couvet D (2001) ESTIM 1.0: a computer program to infer population parameters from one- and two-locus gene identity probabilities. Mol Ecol Notes 1(4):354–356
Belkhir K, Borsa NP, Chikhi L, Raufaste N, Bonhomme F (2004) GENETIX 4.05, Logiciel sousvWindows TM pour la génétique des populations. Laboratoire Génome, Populations, Interactions, CNRS UMR 5000, Université de Montpellier II, Montpellier
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 57(1):289–300
Pouyaud L, Desmarais E, Chenuil A, Agnese JF, Bonhomme F (1999) Kin cohesiveness and possible inbreeding in the mouthbrooding Tilapia Sarotherodon melanotheron (Pisces Cichlidae). Mol Ecol 8(5):803–812
Hassanien HA, Gilbey J (2005) Genetic diversity and differentiation of Nile tilapia (Oreochromis niloticus) revealed by DNA microsatellites. Aquac Res 36(14):1450–1457
Thunken T, Bakker TC, Baldauf SA, Kullmann H (2007) Report active inbreeding in a Cichlid fish and its adaptive significance. Curr Biol 17:225–229
Amoussou TO, Toguyeni A, Imorou Toko I, Chikou A, Akiti T, Enouheran BM, Bogninou CF, Youssao Abdou Karim I (2017) An overview of fish restocking into fresh and brackish inland waterways of Benin (West Africa). Int J Fish Aquat Stud 5(2):164–172
Lederoun D, Chikou A, Vreven E, Snoeks J, Moreau J, Vandewalle P, Lalèyè P (2015) Population parameters and exploitation rate of Sarotherodon melanotheron melanotheron Rüppell, 1852 (Cichlidae) in lake Toho, Benin. J Biodivers Environ Sci 6(2):259–271
Niyonkuru C, Lalèyè PA, Moreau J (2010) Impact of acadja fisheries on the population dynamics of Sarotherodon melanotheron and Hemichromis fasciatus in a lake Nokoué (Benin, West Africa). Knowl Manag Aquat Ecosyst 397(1):1–14
Brookfield JF (1996) A simple new method for estimating null allele frequency from heterozygote deficiency. Mol Ecol 5(3):453–455
Acknowledgements
This research was supported by the International Foundation for Science, Stockholm, Sweden, through a grant to T. Olivier Amoussou (Research Grant No. A/5861-1). T. Olivier Amoussou also benefited from the European Union through the HAAGRIM project (INTRA-ACP Academic Mobility Scheme) and the Government of France through SCAC “Service de Coopération et d’Action Culturelle” (No. 1947/SCAC/JFB and NO. 2015-987732/SCAC/JFB). The authors are grateful to the UEMOA “Union Economique et Monétaire Ouest Africaine” through the PAES/Tilapia-0808 project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed by the authors. The ethical approval for the use of fish in this study was sought and granted from CIRDES’ ethics committee (N/Réf: 04-2015/CE-CIRDES).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amoussou, T.O., Youssao Abdou Karim, I., Dayo, GK. et al. Genetic characterization of Benin’s wild populations of Sarotherodon melanotheron melanotheron Rüppell, 1852. Mol Biol Rep 45, 1981–1994 (2018). https://doi.org/10.1007/s11033-018-4354-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-018-4354-x