Abstract
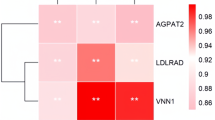
The deposition of intramuscular fat is an important factor affecting the beef quality, such as flavour and palatability. In this study, for further identifying the differential molecular mechanisms regulating the deposition of fat between intramuscular and external adipose tissues, particularly subcutaneous and perirenal adipose tissues, it was designed to obtain transcript sequence data and compare the transcriptomes among intramuscular, subcutaneous, and perirenal adipose tissues by RNA-Seq. A total of 66,206,912, 55,114,070 and 67,320,426 fragments were sequenced for the intramuscular (IAT), subcutaneous (SAT), and perirenal adipose tissue (PAT) respectively. Among them, total 953, 1,534, 2,026 genes showing differential expression between IAT and SAT, IAT and PAT, SAT and PAT, were identified respectively (FDR < 0.05). When these data had been mixed and analyzed together, 110 genes were differentially expressed among these three adipose tissues. Using GO enrichment analysis, multiple biological pathways were found to be significantly enriched for differentially expressed genes (FDR < 0.01), including cellular process, biological regulation, and metabolic process. In addition, total 4,625, 4,775 and 4,147 alternative splicing events occurred in IAT, SAT, and PAT, had also been detected respectively. Thus, our results logically provide the evidence for further understanding the bovine fat deposition, especially intramuscular fat, at a fine scale.




Similar content being viewed by others
References
Hovenier R, Brascamp EW, Kanis E, van der Werf JH, Wassenberg AP (1993) Economic values of optimum traits: the example of meat quality in pigs. J Anim Sci 71(6):1429–1433
Smith SB, Crouse JD (1984) Relative contributions of acetate, lactate and glucose to lipogenesis in bovine intramuscular and subcutaneous adipose tissue. J Nutr 114(4):792–800
Lafontan M, Berlan M (2003) Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci 24(6):276–283
Wajchenberg BL (2000) Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev 21(6):697–738
Bong JJ, Cho KK, Baik M (2010) Comparison of gene expression profiling between bovine subcutaneous and intramuscular adipose tissues by serial analysis of gene expression. Cell Biol Int 34(1):125–133
Zhou G, Wang S, Wang Z, Zhu X, Shu G, Liao W, Yu K, Gao P, Xi Q, Wang X et al (2010) Global comparison of gene expression profiles between intramuscular and subcutaneous adipocytes of neonatal landrace pig using microarray. Meat Sci 86(2):440–450
Wang Z, Gerstein M, Snyder M (2009) RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10(1):57–63
Malone JH, Oliver B (2011) Microarrays, deep sequencing and the true measure of the transcriptome. BMC Biol 9:34
Sultan M, Schulz MH, Richard H, Magen A, Klingenhoff A, Scherf M, Seifert M, Borodina T, Soldatov A, Parkhomchuk D et al (2008) A global view of gene activity and alternative splicing by deep sequencing of the human transcriptome. Science 321(5891):956–960
Lister R, O’Malley RC, Tonti-Filippini J, Gregory BD, Berry CC, Millar AH, Ecker JR (2008) Highly integrated single-base resolution maps of the epigenome in Arabidopsis. Cell 133(3):523–536
Wilhelm BT, Marguerat S, Watt S, Schubert F, Wood V, Goodhead I, Penkett CJ, Rogers J, Bahler J (2008) Dynamic repertoire of a eukaryotic transcriptome surveyed at single-nucleotide resolution. Nature 453(7199):1239–1243
Nagalakshmi U, Wang Z, Waern K, Shou C, Raha D, Gerstein M, Snyder M (2008) The transcriptional landscape of the yeast genome defined by RNA sequencing. Science 320(5881):1344–1349
Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5(7):621–628
Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25(9):1105–1111
Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, Wold BJ, Pachter L (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28(5):511–515
Roberts A, Trapnell C, Donaghey J, Rinn JL, Pachter L (2011) Improving RNA-Seq expression estimates by correcting for fragment bias. Genome Biol 12(3):R22
Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci 95(25):14863–14868
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26(1):139–140
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34(Web Server issue):W293–W297
Foissac S, Sammeth M (2007) ASTALAVISTA: dynamic and flexible analysis of alternative splicing events in custom gene datasets. Nucleic Acids Res 35(Web Server issue):W297–W299
Driver AM, Penagaricano F, Huang W, Ahmad KR, Hackbart KS, Wiltbank MC, Khatib H (2012) RNA-Seq analysis uncovers transcriptomic variations between morphologically similar in vivo- and in vitro-derived bovine blastocysts. BMC Genom 13:118
Balakrishnan CN, Lin YC, London SE, Clayton DF (2012) RNA-seq transcriptome analysis of male and female zebra finch cell lines. Genomics 100(6):363–369
Feng C, Chen M, Xu CJ, Bai L, Yin XR, Li X, Allan AC, Ferguson IB, Chen KS (2012) Transcriptomic analysis of Chinese bayberry (Myrica rubra) fruit development and ripening using RNA-Seq. BMC Genom 13:19
Li S, Wang C, Yu W, Zhao S, Gong Y (2012) Identification of genes related to white and black plumage formation by RNA-Seq from white and black feather bulbs in ducks. PLoS ONE 7(5):e36592
Oberauer R, Rist W, Lenter MC, Hamilton BS, Neubauer H (2010) EGFL6 is increasingly expressed in human obesity and promotes proliferation of adipose tissue-derived stromal vascular cells. Mol Cell Biochem 343(1–2):257–269
Soni KG, Lehner R, Metalnikov P, O’Donnell P, Semache M, Gao W, Ashman K, Pshezhetsky AV, Mitchell GA (2004) Carboxylesterase 3 (EC 3.1.1.1) is a major adipocyte lipase. J Biol Chem 279(39):40683–40689
Nagashima S, Yagyu H, Takahashi N, Kurashina T, Takahashi M, Tsuchita T, Tazoe F, Wang XL, Bayasgalan T, Sato N et al (2011) Depot-specific expression of lipolytic genes in human adipose tissues—association among CES1 expression, triglyceride lipase activity and adiposity. J Atheroscler Thromb 18(3):190–199
Rong S, Cao Q, Liu M, Seo J, Jia L, Boudyguina E, Gebre AK, Colvin PL, Smith TL, Murphy RC et al (2012) Macrophage 12/15 lipoxygenase expression increases plasma and hepatic lipid levels and exacerbates atherosclerosis. J Lipid Res 53(4):686–695
Schmid A, Kopp A, Hanses F, Bala M, Muller M, Schaffler A (2012) The novel adipokine C1q/TNF-related protein-3 is expressed in human adipocytes and regulated by metabolic and infection-related parameters. Exp Clin Endocrinol Diabetes 120(10):611–617
Wei WH, de Koning DJ, Penman JC, Finlayson HA, Archibald AL, Haley CS (2007) QTL modulating ear size and erectness in pigs. Anim Genet 38(3):222–226
Shao GC, Luo LF, Jiang SW, Deng CY, Xiong YZ, Li FE (2011) A C/T mutation in microRNA target sites in BMP5 gene is potentially associated with fatness in pigs. Meat Sci 87(3):299–303
Chavey C, Boucher J, Monthouel-Kartmann MN, Sage EH, Castan-Laurell I, Valet P, Tartare-Deckert S, Van Obberghen E (2006) Regulation of secreted protein acidic and rich in cysteine during adipose conversion and adipose tissue hyperplasia. Obesity 14(11):1890–1897
Nie J, Sage EH (2009) SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem 284(2):1279–1290
Chmurzynska A (2006) The multigene family of fatty acid-binding proteins (FABPs): function, structure and polymorphism. J Appl Genet 47(1):39–48
Samulin J, Berget I, Lien S, Sundvold H (2008) Differential gene expression of fatty acid binding proteins during porcine adipogenesis. Comp Biochem Physiol B: Biochem Mol Biol 151(2):147–152
Taniguchi M, Guan LL, Zhang B, Dodson MV, Okine E, Moore SS (2008) Adipogenesis of bovine perimuscular preadipocytes. Biochem Biophys Res Commun 366(1):54–59
Gardan D, Louveau I, Gondret F (2007) Adipocyte- and heart-type fatty acid binding proteins are both expressed in subcutaneous and intramuscular porcine (Sus scrofa) adipocytes. Comp Biochem Physiol B: Biochem Mol Biol 148(1):14–19
Acknowledgments
This work was supported by Grants from Beijing Natural Science Foundation (Grant No. 6132003), the science and technology project of Beijing Municipal Commission of Education (Grant No. PXM 2014_014207_000001), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (PXM2013_014207_000067), National Natural Science Foundation of China (Grant No. 31272526, Grant No. 31072185), and Beijing University of Agriculture Funding to improve research quality (Grant No. GJB2012002).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xihui Sheng and Hemin Ni have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11033_2013_3010_MOESM1_ESM.xlsx
Table S1. The FPKM value of annotated genes in bovine intramuscular, subcutaneous and perirenal adipose tissues. (XLSX 1034 kb)
Rights and permissions
About this article
Cite this article
Sheng, X., Ni, H., Liu, Y. et al. RNA-seq analysis of bovine intramuscular, subcutaneous and perirenal adipose tissues. Mol Biol Rep 41, 1631–1637 (2014). https://doi.org/10.1007/s11033-013-3010-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-013-3010-8