Abstract
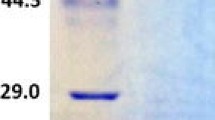
Laccases are strong oxidizing enzymes that oxidize chlorinated phenols, synthetic dyes, pesticides, polycyclic aromatic hydrocarbons as well as a very wide range of other compounds with high redox potential. Based on the bias of genetic codons between fungus and yeast, we synthesized a laccase gene GlLCCI, originated from Ganoderma lucidum using optimized codons and a PCR-based two-step DNA synthesis method. The recombinant laccase, GlLCCI was successfully over-expressed in yeast, Pichia pastoris, with an alcohol oxidase1 promoter. The recombinant GlLCCI has a molecular mass of approximately 58 kDa. The K m values of GlLCCI for 2-2′-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) and guaiacol were 0.9665, and 1.1122 mM, respectively. The V max of GlLCCI for both substrates was 3,024 and 82.13 μM mg−1 min−1. When ABTS was used as a substrate, the enzyme had an optimal temperature of approximately 55°C. The enzyme was detected over pH values from 2 to 8. The enzyme was strongly activated by K+, Na+, Cu2+ and mannitol. Six amino acids (alanine, histidine, glycine, arginine, aspartate and phenylalanine) increased the catalytic ability of the enzyme. The activity of laccase was obviously inhibited by Fe2+, Fe3+, sodium hydrosulphite, and sodium azide. Additionally, under optimal conditions, GlLCCI decolorized 37.62 mg l−1 of azo dye methyl orange (MO) in cultural medium. With a high MO degradation ability, GlLCCI may have potential in the treatment of industrial effluent containing azo dye MO.





Similar content being viewed by others
References
Soden DM, O’Callaghan J, Dobson AD (2002) Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology 148(Pt 12):4003–4014
Thurston CF (1994) The structure and function of fungal laccase. Microbiology 140:19–26
Prillinger H, Esser K (1975) The phenoloxidases of the ascomycete Podospora anserina XIII. Action and interaction of genes controlling the formation of laccase. Mol Gen Genet 156(3):333–345
Childs RE, Bardsley WG (1975) The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem J 145(1):93–103
Murugesan K (2006) Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid state culture. Enzyme Microb Technol 40(2007):1662–1672
Jonsson LJ, Saloheimo M, Penttila M (1997) Laccase from the white-rot fungus Trametes versicolor: cDNA cloning of lcc1 and expression in Pichia pastoris. Curr Genet 32(6):425–430
Wang HX, Ng TB (2006) Purification of a laccase from fruiting bodies of the mushroom Pleurotus eryngii. Appl Microbiol Biotechnol 69(5):521–525. doi:10.1007/s00253-005-0086-7
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30(2):215–242. doi:10.1111/j.1574-4976.2005.00010.x
Paterson RR (2006) Ganode—a therapeutic fungal biofactory. Phytochemistry 67(18):1985–2001. doi:10.1016/j.phytochem.2006.07.004
Gao Y, Gao H, Chan E, Tang W, Xu A, Yang H, Huang M, Lan J, Li X, Duan W, Xu C, Zhou S (2005) Antitumor activity and underlying mechanisms of ganopoly, the refined polysaccharides extracted from Ganoderma lucidum, in mice. Immunol Invest 34(2):171–198
Hseu RS, Wang HH, Wang HF, Moncalvo JM (1996) Differentiation and grouping of isolates of the Ganoderma lucidum complex by random amplified polymorphic DNA-PCR compared with grouping on the basis of internal transcribed spacer sequences. Appl Environ Microbiol 62(4):1354–1363
Joo SS, Ryu IW, Park JK, Yoo YM, Lee DH, Hwang KW, Choi HT, Lim CJ, Lee do I, Kim K (2008) Molecular cloning and expression of a laccase from Ganoderma lucidum, and its antioxidative properties. Mol Cells 25(1):112–118
Ko EM, Leem YE, Choi HT (2001) Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl Microbiol Biotechnol 57(1–2):98–102
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24(1):45–66
Xiong AS, Yao QH, Peng RH, Li X, Fan HQ, Cheng ZM, Li Y (2004) A simple, rapid, high-fidelity and cost-effective PCR-based two-step DNA synthesis method for long gene sequences. Nucleic Acids Res 32(12):e98. doi:10.1093/nar/gnh09432/12/e98
Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H (2002) Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol 60(3):327–335. doi:10.1007/s00253-002-1109-2
Biro JC (2008) Does codon bias have an evolutionary origin? Theor Biol Med Model 5:1–15
Kliman RM, Irving N, Santiago M (2003) Selection conflicts, gene expression, and codon usage trends in yeast. J Mol Evol 57(1):98–109. doi:10.1007/s00239-003-2459-9
Fu XY, Zhao W, Xiong AS, Tian YS, Peng RH (2010) High expression of recombinant Streptomyces sp. S38 xylanase in Pichia pastoris by codon optimization and analysis of its biochemical properties. Mol Biol Rep. doi:10.1007/s11033-010-0644-7
Cereghino GP, Cregg JM (1999) Applications of yeast in biotechnology: protein production and genetic analysis. Curr Opin Biotechnol 10(5):422–427. doi:S0958-1669(99)00004-X
Peter MG, Wollenberger U (1997) Phenol-oxidizing enzymes: mechanisms and applications in biosensors. EXS 80:63–82
Ullrich R, Huong le M, Dung NL, Hofrichter M (2005) Laccase from the medicinal mushroom Agaricus blazei: production, purification and characterization. Appl Microbiol Biotechnol 67(3):357–363. doi:10.1007/s00253-004-1861-6
Wang HX, Ng TB (2004) A novel laccase with fair thermostability from the edible wild mushroom (Albatrella dispansus). Biochem Biophys Res Commun 319(2):381–385. doi:10.1016/j.bbrc.2004.05.011
Wang HX, Ng TB (2004) A new laccase from dried fruiting bodies of the monkey head mushroom Hericium erinaceum. Biochem Biophys Res Commun 322(1):17–21. doi:10.1016/j.bbrc.2004.07.075
Eggert C, Temp U, Eriksson KE (1996) The ligninolytic system of the white rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62(4):1151–1158
Murugesan K, Kim YM, Jeon JR, Chang YS (2009) Effect of metal ions on reactive dye decolorization by laccase from Ganoderma lucidum. J Hazard Mater 168(1):523–529. doi:10.1016/j.jhazmat.2009.02.075
Kiiskinen LL, Viikari L, Kruus K (2002) Purification and characterisation of a novel laccase from the ascomycete Melanocarpus albomyces. Appl Microbiol Biotechnol 59(2–3):198–204. doi:10.1007/s00253-002-1012-x
O’Callaghan J, O’Brien MM, McClean K, Dobson AD (2002) Optimisation of the expression of a Trametes versicolor laccase gene in Pichia pastoris. J Ind Microbiol Biotechnol 29(2):55–59. doi:10.1038/sj.jim.7000268
Acknowledgments
This research study was supported with funding from the Science and Technology Commission of Shanghai Municipality (09dz2200800). Another financial support was the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, J., Peng, RH., Xiong, AS. et al. Secretory expression and characterization of a soluble laccase from the Ganoderma lucidum strain 7071-9 in Pichia pastoris . Mol Biol Rep 39, 3807–3814 (2012). https://doi.org/10.1007/s11033-011-1158-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-1158-7