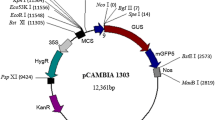
Abstract
Dunaliella salina has been exploited as a new type of bioreactor due to its unique advantages. However, this bioreactor application was restricted for absence of a high-efficiency and stable transformation method at present. In the present study, the cells of D. salina were transformed by glass beads. The results of histochemical staining revealed that the GUS gene was successfully expressed in the positive transformants, and PCR and PCR-Southern blot analysis further demonstrated that the bar gene was integrated into the D. salina genome. Moreover, the three transformation methods, including glass beads, bombardment particle and electroporation, were compared for screening a high-efficiency transformation method for gene engineering of D. salina. The results showed that transformation efficiency of the glass beads was the highest, approximately 102 transformants/μg DNA. It is concluded that the established glass beads method has been demonstrated to be an optimal transformation way for D. salina.








Similar content being viewed by others
Abbreviations
- PEG:
-
Polyethylene glycol
- Nos:
-
Nopaline synthase gene
- PPT:
-
Phosphinothricin
- GUS:
-
Beta-glucuronidase
References
Xue LX, Pan WD, Jiang GZ, Wang JR (2006) Transgenic Dunuliella salina as a bioreactor. Patent No: US 7081567B2
Ben-Amotz A, Sussman I, Avron M (1982) Glycerol production by Dunaliella. Experientia 38:49–52. doi:10.1007/BF01944527
Avron M (1986) The osmotic components of halotolerant algae. Trends Biochem Sci 11:5–6. doi:10.1016/0968-0004(86)90218-5
Sadka A, Himmelhoch S, Zamir A (1991) A 150 Kilodalton cell surface protein is induced by salt in the halotolerant green alga Dunaliella salina. Plant Physiol 95:822–831
Ben-Amotz A, Avron M (1990) The biotechnology of cultivating the halotolerent alga Dunaliella. Trends Biotechnol 8:121–126. doi:10.1016/0167-7799(90)90152-N
Ben-Amotz A, Shaish A, Avron M (1991) The biotechnology of cultivating Dunaliella for production of β-Carotene rich algae. Bioresour Technol 38:233–235. doi:10.1016/0960-8524(91)90160-L
Leach G, Oliveira G, Morais R (1998) Spray-drying of Dunaliella salina to produce a β-Carotene rich power. J Ind Microbiol Biotechnol 20:82–85. doi:10.1038/sj.jim.2900485
Geng DG, Wang YQ, Wang P, Li WB, Sun YR (2003) Stable expression of hepatitis B surface antigen gene in Dunaliella salina (Chlorophyta). J Appl Phycol 15:451–456. doi:10.1023/B:JAPH.0000004298.89183.e5
Boynton JE, Gillham NW, Harris EH, Hosler JP, Johnson AM, Jones AR et al (1988) Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science 240:1534–1538. doi:10.1126/science.2897716
Brown LE, Sprecher SL, Keller LR (1991) Introduction of exogenous DNA into Chlamydomonas reinhardtii by electroporation. Mol Cell Biol 11:2328–2332
Kindle KL (1990) High-frequency nuclear transformation of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 87:1228–1232. doi:10.1073/pnas.87.3.1228
Dunahay TG (1993) Transformation of Chlamydomonas reinhardtii with silicon carbide whiskers. Biotechnology 15:452–460
Kumar SV, Misquitta RW, Reddy VS, Rao BJ, Rajam MV (2004) Genetic transformation of the green alga—Chlamydomonas reinhardtii by Agrobacterium tumefaciens. Plant Sci 166:731–738. doi:10.1016/j.plantsci.2003.11.012
Geng DG, Han Y, Wang YQ, Wang P, Zhang LM, Li WB et al (2004) Construction of a system for the stable expression of foreign genes in Dunaliella salina. Acta Bot Sin 46:342–346
Sun Y, Yang Z, Gao X, Li Q, Zhang Q, Xu Z (2005) Expression of foreign genes in Dunaliella by electroporation. Mol Biotechnol 30:185–192. doi:10.1385/MB:30:3:185
Tan CP, Qin S, Zhang Q, Jiang P, Zhao GQ (2005) Establishment of a micro-particle bombardment transformation system for Dunaliella salina. Microbiology 43:361–365
Costanzo MC, Fox TD (1988) Transformation of yeast by agitation with glass beads. Genetics 120:667–670
Xie H, Xu PR, Jia YL, Li J, Lu ZM, Xue LX (2007) Cloning and heterologous expression of nitrate reductase genes from Dunaliella salina. J Appl Phycol 19:497–504. doi:10.1007/s10811-007-9162-y
Ben-Amotz A, Avron M (1983) Accumulation of metabolites by halotolerent algae and its industrial potential. Annu Rev Microbiol 37:95–119. doi:10.1146/annurev.mi.37.100183.000523
Jefferson RA, Knvanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Chen Y, Wang Y, Sun Y, Zhang L, Li W (2001) Highly efficient expression of rabbit neutrophil peptide-1 gene in Chlorella ellipsoidea cells. Curr Genet 39:365–370. doi:10.1007/s002940100205
Shimogawara K, Fujiwara S, Grossman A, Usuda H (1998) High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics 148:1821–1828
Acknowledgements
This work was supported by the grants from International Science and Technology Cooperation Program of The Ministry of Science and Technology of P.R. China (No. 2007DFA01240), Special Foundation for Training of Doctoral Students from Institutions of Higher Learning, Ministry of Education of P.R. China (No. 20050459007) and National Natural Science Foundation of China (No. 30600006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, S., Xue, L., Liu, H. et al. Improvement of efficiency of genetic transformation for Dunaliella salina by glass beads method. Mol Biol Rep 36, 1433–1439 (2009). https://doi.org/10.1007/s11033-008-9333-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-008-9333-1