Abstract
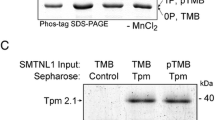
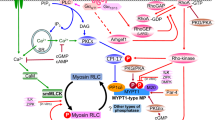
In this study, we provide further insight into the contribution of the smoothelin-like 1 (SMTNL1) calponin homology (CH)-domain on myosin light chain phosphatase (SMPP-1M) activity and smooth muscle contraction. SMTNL1 protein was shown to have inhibitory effects on SMPP-1M activity but not on myosin light chain kinase (MLCK) activity. Treatment of β-escin permeabilized rabbit, ileal smooth muscle with SMTNL1 had no effect on the time required to reach half-maximal force (t1/2) during stimulation with pCa6.3 solution. The addition of recombinant SMTNL1 protein to permeabilized, smooth muscle strips caused a significant decrease in contractile force. While the calponin homology (CH)-domain was essential for maximal SMTNL1-associated relaxation, it alone did not cause significant changes in force. SMTNL1 was poorly dephosphorylated by PP-1C in the presence of the myosin targeting subunit (MYPT1), suggesting that phosphorylated SMTNL1 does not possess “substrate trapping” properties. Moreover, while full-length SMTNL1 could suppress SMPP-1M activity toward LC20 in vitro, truncated SMTNL1 lacking the CH-domain was ineffective. In summary, our findings suggest an important role for the CH-domain in mediating the effects of SMTNL1 on smooth muscle contraction.





Similar content being viewed by others
References
Borman MA, MacDonald JA, Haystead TA (2004) Modulation of smooth muscle contractility by CHASM, a novel member of the smoothelin family of proteins. FEBS Lett 573:207–213. doi:10.1016/j.febslet.2004.08.002
Wooldridge AA, Fortner CN, Lontay B, Akimoto T, Neppl RL, Facemire C, Datto MB, Kwon A, McCook E, Li P, Wang S, Thresher RJ, Miller SE, Perriard JC, Gavin TP, Hickner RC, Coffman TM, Somlyo AV, Yan Z, Haystead TAJ (2008) Deletion of the protein kinase A/protein kinase G target SMTNL1 promotes an exercise-adapted phenotype in vascular smooth muscle. J Biol Chem 283:11850–11859. doi:10.1074/jbc.M708628200
Somlyo AP, Somlyo AV (2003) Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev 83:1325–1358
Schlossmann J, Feil R, Hofmann F (2003) Signaling through NO and cGMP-dependent protein kinases. Ann Med 35:21–27. doi:10.1080/07853890310004093
Ishida H, Borman MA, Ostrander J, Vogel HJ, MacDonald JA (2008) Solution structure of the calponin homology (CH) domain from the smoothelin-like 1 protein: a unique apocalmodulin-binding mode and the possible role of the C-terminal type-2 CH-domain in smooth muscle relaxation. J Biol Chem 283:20569–20578. doi:10.1074/jbc.M800627200
Lorenzi M, Gimona M (2008) Synthetic actin-binding domains reveal compositional constraints for function. Int J Biochem Cell Biol 40:1806–1816. doi:10.1016/j.biocel.2008.01.011
Friedberg F (2008) Alternative splicing for members of human mosaic domain superfamilies. I. The CH and LIM domains containing group of proteins. Mol Biol Rep. doi:10.1007/s11033-008-9281-9
Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ (2002) Functional plasticity of CH domains. FEBS Lett 513:98–106. doi:10.1016/S0014-5793(01)03240-9
Ihara E, Moffat L, Ostrander J, Walsh MP, MacDonald JA (2007) Characterization of protein kinase pathways responsible for Ca2+ sensitization in rat ileal longitudinal smooth muscle. Am J Physiol Gastrointest Liver Physiol 293:G699–G710. doi:10.1152/ajpgi.00214.2007
Wu X, Somlyo AV, Somlyo AP (1996) Cyclic GMP-dependent stimulation reverses G-protein-coupled inhibition of smooth muscle myosin light chain phosphate. Biochem Biophys Res Commun 220:658–663. doi:10.1006/bbrc.1996.0460
Kitazawa T, Masuo M, Somlyo AP (1991) G protein-mediated inhibition of myosin light-chain phosphatase in vascular smooth muscle. Proc Natl Acad Sci USA 88:9307–9310. doi:10.1073/pnas.88.20.9307
MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TAJ (2001) Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci USA 98:2419–2424. doi:10.1073/pnas.041331498
Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP (1998) Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J Biol Chem 273:11362–11369. doi:10.1074/jbc.273.18.11362
Woodrum D, Pipkin W, Tessier D, Komalavilas P, Brophy CM (2003) Phosphorylation of the heat shock-related protein, HSP20, mediates cyclic nucleotide-dependent relaxation. J Vasc Surg 37:874–881. doi:10.1067/mva.2003.153
Walker LA, MacDonald JA, Liu X, Nakamoto RK, Haystead TA, Somlyo AV, Somlyo AP (2001) Site-specific phosphorylation and point mutations of telokin modulate its Ca2+-desensitizing effect in smooth muscle. J Biol Chem 276:24519–24524. doi:10.1074/jbc.M103560200
Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA (2004) Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem 279:34496–34504. doi:10.1074/jbc.M405957200
Nakamura K, Koga Y, Sakai H, Homma K, Ikebe M (2007) cGMP-dependent relaxation of smooth muscle is coupled with the change in the phosphorylation of myosin phosphatase. Circ Res 101:712–722. doi:10.1161/CIRCRESAHA.107.153981
Quensel C, Kramer J, Cardoso MC, Leonhardt H (2002) Smoothelin contains a novel actin cytoskeleton localization sequence sequence with similarity to troponin T. J Cell Biochem 85:403–409. doi:10.1002/jcb.10143
Niessen P, Clement S, Fontao L, Caponnier C, Teunissen B, Rensen S, van Eys G, Gabbiani G (2004) Biochemical evidence for interaction between smoothelin and filamentous actin. Exp Cell Res 292:170–178. doi:10.1016/j.yexcr.2003.09.005
Zhang W, Wu Y, Du L, Tang DD, Gunst SJ (2005) Activation of the Arp2/3 complex by N-WASp is required for actin polymerization and contraction in smooth muscle. Am J Physiol Cell Physiol 288:C1145–C1160. doi:10.1152/ajpcell.00387.2004
Acknowledgments
This work was supported by research grants from the Heart and Stroke Foundation of Canada (HSFC) to J.A.M. and National Institutes of Health (DK065954-02) to T.A.J.H. M.A.B. was a recipient of Fellowships from the Alberta Heritage Foundation for Medical Research (AHFMR) and HSFC. J.A.M. is recipient of a Canada Research Chair (Tier II) in Smooth Muscle Pathophysiology and a Scholarship from HSFC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borman, M.A., Freed, T.A., Haystead, T.A.J. et al. The role of the calponin homology domain of smoothelin-like 1 (SMTNL1) in myosin phosphatase inhibition and smooth muscle contraction. Mol Cell Biochem 327, 93–100 (2009). https://doi.org/10.1007/s11010-009-0047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-009-0047-z