Abstract
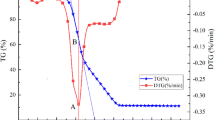
The combustion kinetics of waste capsicum stalk (WCS) in Western China is investigated through thermogravimetric analysis compared with sawdust and coal, and co-combustion of WCS with coal is also investigated. Results show that the ignition characteristics of WCS is better than that of sawdust and coal, and the activation energy E of WCS-volatile combustion and WCS-char combustion are 78.55 kJ mol−1 and 44.59 kJ mol−1. However, integrating the characteristics of ignition and burnout, the combustion characteristic factor (S N) of WCS is lower than that of sawdust. With the increasing in the heating rate, the ignition of WCS is delayed. Oxygen concentration \( C_{{{\text{O}}_{2} }} \) affects E and k 0 of volatile combustion largely under rich-oxygen condition, when \( C_{{{\text{O}}_{2} }} \) increases from 0.2 to 0.8, E has increased threefold and k 0 also intensively increases from 106 to 1013–1022. Oppositely, effect of \( C_{{{\text{O}}_{2} }} \) on the E and k 0 of char combustion is little, and there is an exponential relationship \( S_{\text{N}} = 7. 1 2 8 \times 10^{ - 9} \times { \exp }(C_{{{\text{O}}_{2} }} /0. 3 6 8) - 6. 1 2 6 \times 10^{ - 9} \) between S N and \( C_{{{\text{O}}_{2} }} \). For the tests of co-combustion, all the experimental and weighted-average curves coincide well, and there is no remarkable synergistic effect. With the increase of mixing ratio that WCS added, E and k 0 of volatile combustion increase, but correspondingly E and k 0 of char combustion decrease.











Similar content being viewed by others
References
Wang XB, et al. Nitrogen, sulfur, and chlorine transformations during the pyrolysis of straw. Energy Fuels. 2010;24:5215–21.
Dumanli AG, Tas S, Yurum Y. Co-firing of biomass with coals Part 1. Thermogravimetric kinetic analysis of combustion of fir (Abies bornmulleriana) wood. J Therm Anal Calorim. 2011;103(3):925–33.
Wang X-B, Zhao Q-X, Tan H-Z. Kinetic analysis of nitric oxide reduction using biogas as reburning fuel. Afr J Biotechnol. 2009;8(10):2251–7.
Gonzalez-Diaz L, et al. Expert system for integrated plant protection in pepper (Capsicum annuun L.). Exp Syst Appl. 2009;36:8975–9.
Kulkarni M, Phalke S. Evaluating variability of root size system and its constitutive traits in hot pepper (Capsicum annum L.) under water stress. Sci Hortic. 2009;120:159–66.
Yongxia H, Baoli Z, Xiaoling W. Allelopathy of decomposing pepper stalk on pepper growth. Chin J Appl Ecol. 2006;17(4):699–702.
Wang X, et al. Experimental investigation on biomass co-firing in a 300 MW pulverized coal-fired utility furnace in China. Proc Combust Inst. 2011;33(2):2725–33.
Ulloa CA, Gordon AL, García XA. Thermogravimetric study of interactions in the pyrolysis of blends of coal with radiata pine sawdust. Fuel Process Technol. 2009;90:583–90.
Wei X, Lopez C, Puttkamer Tv. Assessment of chlorine-alkali-mineral interactions during co-combustion of coal and straw. Energy Fuels. 2002;16:1095–108.
Tan HZ, et al. Influence of metal elements on the evolution of CO and CH4 during the pyrolysis of sawdust. Afr J Biotechnol. 2010;9(3):331–9.
Morgana PA, Robertsona SD, Unswortha JF. Combustion studies by thermogravimetric analysis: 1. Coal Oxid Fuel. 1986;65(11):1546–51.
Zhao Y, Hu H, Jin L. Pyrolysis behavior of weakly reductive coals from northwest China. Energy Fuels. 2009;23:870–5.
Ghetti P, Robertis UD, D’Antone S. Coal combustion: correlation between surface area and thermogravimetric analysis data. Fuel. 1985;64(7):950–5.
Suarez A, et al. Thermal analysis of the combustion of charcoals from Eucalyptus dunnii obtained at different pyrolysis temperatures. J Therm Anal Calorim. 2010;100(3):1051–4.
Niu SL, et al. Thermogravimetric analysis of combustion characteristics and kinetic parameters of pulverized coals in oxy-fuel atmosphere. J Therm Anal Calorim. 2009;98(1):267–74.
Ren Y, Freitag NP, Mahinpey N. A simple kinetic model for coke combustion during an in situ combustion (ISC) process. J Can Petrol Technol. 2007;46(4):47–53.
Ambalae A, Mahinpey N, Freitag N. Thermogravimetric studies on pyrolysis and combustion behavior of a heavy oil and its asphaltenes. Energy Fuels. 2006;20(2):560–5.
Zouaoui N, et al. Study of experimental and theoretical procedures when using thermogravimetric analysis to determine kinetic parameters of carbon black oxidation. J Therm Anal Calorim. 2010;102(3):837–49.
Kalogirou M, Samaras Z. Soot oxidation kinetics from TG experiments. J Therm Anal Calorim. 2010;99(3):1005–10.
Sancheza ME, et al. Thermogravimetric kinetic analysis of the combustion of biowastes. Renew Energy. 2009;34(6):1622–7.
Li X, Ma B, Xua L. Thermogravimetric analysis of the co-combustion of the blends with high ash coal and waste tyres. Thermochim Acta. 2006;441(4):79–83.
García AN, Font R, Esperanza MM. Thermogravimetric kinetic model of the combustion of a varnish waste based on polyurethane. Energy Fuels. 2001;15(4):848–55.
Zhaosheng Y, Xiaoqian M, Ao L. Thermogravimetric analysis of rice and wheat straw catalytic combustion in air- and oxygen-enriched atmospheres. Energy Convers Manag. 2009;50:561–6.
Zapata B, et al. Thermo-kinetics study of orange peel in air. J Therm Anal Calorim. 2009;98(1):309–15.
Yagmur S, Durusoy T. Kinetics of combustion of oil shale with polystyrene. J Therm Anal Calorim. 2009;96(1):189–94.
Ferdous D, Dalai AK, Bej SK. Pyrolysis of lignins: experimental and kinetics studies. Energy Fuels. 2002;16(6):1405–12.
Cloke M, Lester E, Leney M. Effect of volatile retention on products from low temperature pyrolysis in a fixed bed batch reactor. Fuel. 1999;78:1719–28.
Matham JG, Kandiyoti R. Coal pyrolysis yields from fast and slow heating in a wire-mesh apparatus with a gas sweep. Energy Fuels. 1988;2(4):505–11.
Buryan P, Staff M. Pyrolysis of the waste biomass. J Therm Anal Calorim. 2008;93:637–40.
Şahin Ö, Özdemir M, Aslanolu M. Calcination kinetics of ammonium pentaborate using the Coats-Redfern and genetic algorithm method by thermal analysis. Ind Eng Chem Res. 2001;40(6):1465–70.
Kok MV, Keskin C. Comparative combustion kinetics for in situ combustion process. Thermochim Acta. 2001;369(1):143–7.
Coats A, Redfern J. Kinetic parameters from thermogravimetric data. Nature. 1964;201:68–9.
Nie QH, Sun SZ, Li ZQ. Thermogravimetric analysis on the combustion characteristics of brown coal blends. J Combust Sci Technol. 2001;7(1):72–6.
Wang C, FengyinWang QirongYang. Thermogravimetric studies of the behavior of wheat straw with added coal during combustion. Biomass Bioenergy. 2009;33:50–6.
Tsamba AJ, Yang W, Blasiak W. Pyrolysis characteristics and global kinetics of coconut and cashew nut shells. Fuel. 2006;86:523–30.
Liang XH, Kozinski JA. Numerical modeling of combustion and pyrolysis of cellulosic biomass in thermogravimetric systems. Fuel. 2000;79:1477–86.
Yu LJ, Wang S, Jiang XM. Thermal analysis studies on combustion characteristics of seaweed. J Therm Anal Calorim. 2008;93(2):611–7.
Zhou L, Wang Y, Huang Q. Thermogravimetric characteristics and kinetic of plastic and biomass blends co-pyrolysis. Fuel Process Technol. 2006;87:963–9.
Sutcu H. Pyrolysis by thermogravimetric analysis of blends of peat with coals of different characteristics and biomass. J Chin Inst Chem Eng. 2007;38:245–9.
Orfao JJM, Antunes FJA, Figueiredo JL. Pyrolysis kinetics of lignocellulosic materials—three independent reactions model. Fuel. 1999;78:349–58.
Biagini E, Lippi F, Petarca L. Devolatilization rate of biomasses and coal-biomass blends: an experimental investigation. Fuel. 2002;81:1041–50.
Yang H, Yan R, Chen H. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
McKendry P. Energy production from biomass (part 1): overview of biomass. Bioresour Technol. 2002;83:37–46.
Gani A, Naruse I. Effect of cellulose and lignin content on pyrolysis and combustion characteristics for several types of biomass. Renew Energy. 2007;32:649–61.
Janse AMC, de Jonge HG, Prins W. The combustion kinetics of char obtained by flash pyrolysis of pine wood. Ind Eng Chem Res. 1998;37:3909–18.
Varhegyi G, Meszaros E, Antal M. Combustion kinetics of corncob charcoal and partially demineralized corncob charcoal in the kinetic regime. Ind Eng Chem Res. 2006;45:4962–70.
Kashiwagi T, Nambu H. Global kinetic constants for thermal oxidative degradation of a cellulosic sample. Combust Flame. 1992;88:345–68.
Fang MX, Shen DK, Li YX. Kinetic study on pyrolysis and combustion of wood under different oxygen concentrations by using TG-FTIR analysis. J Anal Appl Pyrolysis. 2006;77:22–7.
Quan C, Li AM, Gao NB. Thermogravimetric analysis and kinetic study on large particles of printed circuit board wastes. Waste Manage. 2009;29(8):2353–60.
Chan WCR, Kelbon M, Krieger-Brockett B. Single-particle biomass pyrolysis: correlations of reaction products with process conditions. Ind Eng Chem Res. 1988;27(12):2261–75.
Encinar JM, et al. Pyrolysis of two agricultural residues: olive and grape bagasse. Influence of particle size and temperature. Biomass Bioenergy. 1996;11(5):397–409.
Zhang X, et al. Study on biomass pyrolysis kinetics. J Eng Gas Turbines Power. 2006;128(3):493–6.
Aboulkas A, et al. Pyrolysis kinetics of olive residue/plastic mixtures by non-isothermal thermogravimetry. Fuel Process Technol. 2009;90(5):722–8.
Vuthaluru HB. Investigations into the pyrolytic behaviour of coal/biomass blends using thermogravimetric analysis. Bioresour Technol. 2004;92(2):187–95.
Sonobe T, Worasuwannarak N, Pipatmanomai S. Synergies in co-pyrolysis of Thai lignite and corncob. Fuel Process Technol. 2008;89(12):1371–8.
Garc I, et al. Co-pyrolysis of sugarcane bagasse with petroleum residue. Part I: thermogravimetric analysis. Fuel. 2001;80(9):1245–58.
Sharypov VI, et al. Co-pyrolysis of wood biomass and synthetic polymer mixtures. Part I: influence of experimental conditions on the evolution of solids, liquids and gases. J Anal Appl Pyrolysis. 2002;64(1):15–28.
Kastanaki E, Vamvuka D. A comparative reactivity and kinetic study on the combustion of coal-biomass char blends. Fuel. 2006;85(9):1186–93.
Haykiri-Acma H, Yaman S. Effect of biomass on burnouts of Turkish lignites during co-firing. Energy Convers Manag. 2009;50(9):2422–7.
Acknowledgements
The present work was supported by the Natural Science Funds of China (No. 50976086), ESPRC (EP/F061188/1), and China Scholarship Council.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Si, J., Tan, H. et al. Kinetics investigation on the combustion of waste capsicum stalks in Western China using thermogravimetric analysis. J Therm Anal Calorim 109, 403–412 (2012). https://doi.org/10.1007/s10973-011-1556-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1556-z