Abstract
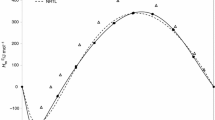
Excess molar enthalpies for two ternary mixtures of {x 1 tributylphosphate (TBP) + x 2 water + x 3 methanol/ethanol} were measured at T = 298.15 K and atmospheric pressure using a TAM Air isothermal calorimeter, by mixing methanol or ethanol with binary mixtures of (water + TBP). Excess enthalpies for initial binary mixtures of (water + TBP) were also measured under the same conditions, which showed phase separation at low molar fraction of TBP. Experimental results of the ternary mixtures were expressed with constant excess molar enthalpy contours on Roozeboon diagrams.



Similar content being viewed by others
References
Laintz KE, Tachikawa E. Extraction of lanthanides from acidic solution using tributyl phosphate modified supercritical carbon dioxide. Anal Chem. 1994;66:2170–93.
Mincher BJ, Martin LR, Schmitt NC. Tributylphosphate extraction behavior of bismuthate-oxidized americium. Inorg Chem. 2008;47:6984–9.
Chiarizia R, Jensen MP, Richert PG, Kolarik Z, Borkowski M, Thiyagarajan P. Extraction of zirconium nitrate by TBP in n-octane: influence of cation type on third phase formation according to the “Sticky Spheres” model. Langmuir. 2004;20:10798–808.
Fox RV, Ball RD, Harrington PB, Rollins HW, Jolley JJ, Wai CM. Praseodymium nitrate and neodymium nitrate complexation with organophorus reagents in supercritical carbon dioxide solvent. J Supercrit Fluids. 2004;31:273–86.
Shamsipur M, Ghiasvand AR, Yamini Y. Extration of uranium from solid matrices using modified supercritical fluid CO2. J Supercrit Fluids. 2001;20:163–9.
Tomioka O, Enokida Y, Yamamoto I, Takahashi T. Cleaning of materials contaminated with metal oxides through supercritical fluid extraction with CO2 containing TBP. Prog Nuclear Energy. 2000;37:417–22.
Shimizu R, Sawada K, Enokida Y, Yamamoto I. Supercritical fluid extraction of rare earth elements from luminescent material in waste fluorescent lamps. J Supercrit Fluids. 2005;33:235–41.
Yonker CR, Smith RD. Stationary phase salvation in capillary supercritical fluid chromatography. Anal Chem. 1989;61:1348–53.
Inada Y, Horita T, Yokooka Y, Funahashi S. Kinetic effect of cosolvents on the metalation reation of porphyrin with bis(β-diketonato)copper(ΙΙ) in supercritical carbon dioxide. J Supercrit Fluids. 2004;31:175–83.
Russick EM, Poulter GA, Adkins CLJ, Sorensen NR. Corrosive effects of supercritical carbon dioxide and cosolvents on metals. J Supercrit Fluids. 1996;9:43–50.
Liu SJ, Inada Y, Funahashi S. Kinetic study of the metalation reaction of 5,10,15,20-tetrakis (pentafluorophenyl)prophyrin with water and methanol adducts of bis(1,1,1,5,5,5-hexafluoropentane-2,4-dionato)zinc(II) in supercritical carbon dioxide: specific cosolvent effect on the metalation. J Supercrit Fluids. 2004;30:237–46.
Smith RM. Supercritical fluids in separation science–the dreams, the rality and the future. J Chromatograph A. 1999;856:83–115.
Liu SJ, Liu HL, Xiao ZL, Chen Q. Y and Yang D W. Molar excess enthalpies of binary mixtures of [tributyl phosphate + {CH3(CH2)nOH (n = 0 to 3)}] at 298.15 K. J Chem Thermodyn. 2007;39:412–6.
Liu HL, Liu SJ, Xiao ZL, Chen QY, Yang DW. Excess molar enthalpies of binary mixtures for (tributyl phosphate + methanol/ethanol) at 298.15 K. J Therm Anal Calorim. 2006;85:541–4.
Peng DY, Benson GC, Lu BCY. Excess enthalpies of cyclohexane + n-hexane + (n-octane or n-dodecane) ternary mixture at 298.15 K. Fluid Phase Equilibria. 2000;171:105–13.
Feng S (1995) Handbook of fine chemicals. Guangdong: Guangdong Science and Technology Press (Chinese), 1995.
Simonson JM, Bradley DJ, Busey RH. Excess molar enthalpies and the thermodynamics of (methanol + water) to 573 K and 40 MPa. J Chem Thermodyn. 1987;19:479–92.
Landgren M, McEachern D, Olofsson Q, Randzio S, Sunner S. Evaluation of excess enthalpies from flow-calorimetric measurements of enthalpies of dilution using local approximation by polynomials. J Chem Thermodyn. 1978;10:847–54.
Morris JW, Mulvey PJ, Abbott MM, Van Ness HC. Excess thermodynamic functions for ternary systems. I. Acetone-chloroform-methanol at 50 °C. J Chem Eng Data. 1975;20:403–5.
Nagata I, Tamura K, Książczak A. Excess enthalpies for (methanol, or propan-1-ol + acetonitrile + propanone) at the temperature 298.15 K. J Chem Thermodyn. 1999;31:491–6.
Acknowledgements
Project supported by the National Natural Science Foundation of China (20873182).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, HL., Liu, SJ. & Chen, QY. Excess molar enthalpies for ternary mixtures of (methanol or ethanol + water + tributyl phosphate) at T = 298.15 K. J Therm Anal Calorim 107, 837–841 (2012). https://doi.org/10.1007/s10973-011-1456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-011-1456-2