Abstract
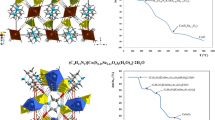
The thermal properties of a series of the six copper selenide cluster molecules [Cu26Se13(PEt2R)14] (R = Ph, Et), [Cu44Se22(PEt2Ph)18], [Cu70Se35(PEt2R)23] (R = Ph, Et) and [Cu140Se70(PEt3)34] have been investigated along with a characterization of their thermolysis products. For all cluster molecules the phosphine ligand shells are cleaved at temperatures between 60°C and 200°C depending on the experimental conditions (Helium gas flow or vacuum), the type of phosphine ligand and the size of the cluster molecules. The residues of the thermal treatment to 150°C were found to be nanostructured Cu2Se with crystallite sizes of approximately 12–16 nm which means that the 1–2.5 nm sized cluster cores of the precursor cluster molecules simultaneously grow during this process. A mixture of processes and factors including the strength of the Cu–P bond, the boiling point of the phosphine ligand as well as the thermal stability of the copper selenide clusters against formation of the bulk material determine the shape of the TGA curves. We found some indications in the TGA along with differential scanning calorimetry (DSC), which suggest that the cleavage of the phosphine ligands is probably mostly determined by the tendency of the metastable clusters to form the bulk material meaning their thermodynamic stability and not by the strength of the Cu–P bond. The data for the series of PEt3 ligated clusters reveal that this stability is dependent on the size of the cluster molecules.







Similar content being viewed by others
References
G. Schmid (eds) (2004) Nanoparticles: From Theory to Application. Wiley-VCH, Weinheim
Schmid G. (eds) (1995) Clusters and Colloids. VCH, Weinheim
H. Krautscheid, D. Fenske, G. Baum, and M. Semmelmann (1993). Angew. Chem. 105, 1364; (1993) Angew. Chem. Int. Ed. Engl. 32, 1303
D. Fenske, N. Zhu, and T. Langetepe (1998). Angew. Chem. 110, 2783; (1998) Angew. Chem. Int. Ed. Engl. 37, 2639
X.-J. Wang, T. Langetepe, C. Persau, B.-S. Kang, G. M. Sheldrick, and D. Fenske (2002). Angew. Chem. 114, 3972; (2002) Angew. Chem. Int. Ed. Engl. 41, 3818
D. Fenske, C. Persau, S. Dehnen, and Ch. A. Anson (2004). Angew. Chem. 116, 308; (2004) Angew. Chem. Int. Ed. Engl. 43, 305
S. Dehnen, A. Schäfer, D. Fenske, and R. Ahlrichs (1994). Angew. Chem. 106, 786; (1994) Angew. Chem. Int. Ed. Engl. 33, 746
Schäfer A., Ahlrichs R. (1994) J. Am. Chem. Soc. 116: 10686
Dehnen S., Schäfer A., Ahlrichs R., Fenske D. (1996) Chem. Eur. J. 2: 429
J. F.Corrigan and D. Fenske (1997). Angew. Chem. 109, 2070; (1997) Angew. Chem. Int. Ed. Engl. 36, 1981
A. Eichhöfer and E. Tröster (2002). Eur. J. Inorg. Chem. 2253
A. Eichhöfer and P. Deglmann (2004). Eur. J. Inorg. Chem. 349
Eichhöfer A., Beckmann E., Fenske D., Herein D., Krautscheid H., Schlögl R. (2001) Isr. J. Chem. 41: 31
van der Putten D., Olevano D., Zanoni R., Krautscheid H., Fenske D. (1995) J. Electron. Spectrosc. Relat. Phenom. 76: 207–211
A. Deveson, S. Dehnen, and D. Fenske (1997). J. Chem. Soc., Dalton Trans. 4491
S. Dehnen and D. Fenske (1994). Angew. Chem. 106, 2369; (1994) Angew. Chem. Int. Ed. Engl. 33, 2287
D. Fenske and H. Krautscheid (1990). Angew. Chem. 102, 1513; (1990) Angew. Chem. Int. Ed. Engl. 29, 1452
N. Zhu and D. Fenske (1999). J. Chem. Soc., Dalton Trans. 1067
S. Dehnen, A. Eichhöfer, and D. Fenske (2002). Eur. J. Inorg. Chem. 279
Leyssens T., Peeters D., Guy Orpen A., Harvey J. N. (2005) New J. Chem. 29: 1424
A. Eichhöfer, D. Fenske, and P. Scheer (2004). Eur. J. Inorg. Chem. 93
In Powder diffraction file PDF-2 Database Sets 1–85, 1993, International Center for Diffraction Data, Newtown Square USA, File number 27-1131; A. L. N. Stevels, Philips Res. Rep. Suppl. 1969,9, 39–44
Kashida S., Akai J. (1988) J. Phys. C: Solid State Phys. 21: 5329
Yamamoto K., Kashida S. (1991) J. Solid State Chem. 93: 202
D. A. Edwards and R. Richards (1997). J. Chem. Soc. Dalton Trans. 4491
Schmidt H., Ruf H. (1963) Z. Anorg. Allg. Chem. 321: 270
K. Sasse, Methoden der Organischen Chemie, Band 1, Houben-Weyl (ed.) (Thieme Verlag, Stuttgart, 1963), p. 32; H. D. Kaesz and F. G. Stone (1959). J. Org. Chem. 24, 635
E. Keller, SCHAKAL 97, A Computer Program for the Graphic Representation of Molecular and Crystallographic Models, Universität Freiburg, 1997
Peerson O. B., Wu X., Kustanovich I, Smith S. O. (1993) J. Magn. Reson., Ser. A 104(3): 33
A. E. Bennett, C. M. Rienstra, M. Auger, K. V. Lakshme, and R. G. Griffen (1995). J. Chem. Phys. 103, 6951
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (Center of functional nanostructures) and the Natural Sciences and Engineering Research Council of Canada (JFC). We thank Frau Tröster for her valuable assistance in the laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. G. Schmid on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Cave, D., Corrigan, J.F., Eichhöfer, A. et al. Investigation of the Thermal Properties of a Series of Copper Selenide Cluster Molecules. J Clust Sci 18, 157–172 (2007). https://doi.org/10.1007/s10876-006-0093-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-006-0093-6