Abstract
Purpose
This study aimed to characterize the clinical phenotype, genetic basis, and consequent immunological phenotype of a boy with severe infantile-onset colitis and eosinophilic gastrointestinal disease, and no evidence of recurrent or severe infections.
Methods
Trio whole-exome sequencing (WES) was utilized for pathogenic variant discovery. Western blot (WB) and immunohistochemical (IHC) staining were used for protein expression analyses. Immunological workup included in vitro T cell studies, flow cytometry, and CyTOF analysis.
Results
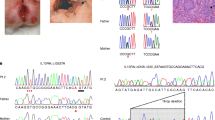
WES revealed a homozygous variant in the capping protein regulator and myosin 1 linker 2 (CARMIL2) gene: c.1590C>A; p.Asn530Lys which co-segregated with the disease in the nuclear family. WB and IHC analyses demonstrated reduced protein levels in patient’s cells compared with controls. Moreover, comprehensive immunological workup revealed severely diminished blood-borne regulatory T cell (Treg) frequency and impaired in vitro CD4+ T cell proliferation and Treg generation. CyTOF analysis showed significant shifts in the patient’s innate and adaptive immune cells compared with healthy controls and ulcerative colitis patients.
Conclusions
Pathogenic variants in CARMIL2 have been implicated in an immunodeficiency syndrome characterized by recurrent infections, occasionally with concurrent chronic diarrhea. We show that CARMIL2-immunodeficiency is associated with significant alterations in the landscape of immune populations in a patient with prominent gastrointestinal disease. This case provides evidence that CARMIL2 should be a candidate gene when diagnosing children with very early onset inflammatory and eosinophilic gastrointestinal disorders, even when signs of immunodeficiency are not observed.





Similar content being viewed by others
References
Uhlig HH, Schwerd T, Koletzko S, Shah N, Kammermeier J, Elkadri A, et al. The diagnostic approach to monogenic very early onset inflammatory bowel disease. Gastroenterology. 2014;147:990–1007.
Kammermeier J, Dziubak R, Pescarin M, Drury S, Godwin H, Reeve K, et al. Phenotypic and genotypic characterisation of inflammatory bowel disease presenting before the age of two years. J Crohn's Colitis. 2017;11:60–9.
Uhlig HH, Schwerd T. From genes to mechanisms: the expanding spectrum of monogenic disorders associated with inflammatory bowel disease. Inflamm Bowel Dis. 2016;22:202–12.
Schober T, Magg T, Laschinger M, Rohlfs M, Linhares ND, Puchalka J, et al. A human immunodeficiency syndrome caused by mutations in CARMIL2. Nat Commun. 2017;8:14209.
Sorte HS, Osnes LT, Fevang B, Aukrust P, Erichsen HC, Backe PH, et al. A potential founder variant in CARMIL2/RLTPR in three Norwegian families with warts, molluscum contagiosum, and T-cell dysfunction. Mol Genet genomic Med. 2016;4:604–16.
Wang Y, Ma CS, Ling Y, Bousfiha A, Camcioglu Y, Jacquot S, et al. Dual T cell– and B cell–intrinsic deficiency in humans with biallelic RLTPR mutations. J Exp Med. 2016;213:2413–35.
Alazami AM, Al-Helale M, Alhissi S, Al-Saud B, Alajlan H, Monies D, et al. Novel CARMIL2 mutations in patients with variable clinical dermatitis, infections, and combined immunodeficiency. Front Immunol. 2018;9:203.
Roncagalli R, Cucchetti M, Jarmuzynski N, Grégoire C, Bergot E, Audebert S, et al. The scaffolding function of the RLTPR protein explains its essential role for CD28 co-stimulation in mouse and human T cells. J Exp Med. 2016;213:2437–57.
Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017;45:D840–5.
Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, et al. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res. 2001;29:308–11.
The 1000 Genomes Project Consortium. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012;491:56–65.
Scott EM, Halees A, Itan Y, Spencer EG, He Y, Abdellateef Azab M, et al. Characterization of greater middle eastern genetic variation for enhanced disease gene discovery. Nat Genet. 2016;48:1071–6.
Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat Protoc. 2009;4:1073–82.
Schwarz JM, Cooper DN, Schuelke M, Seelow D. Mutationtaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–2.
Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–9.
Ashkenazy H, Abadi S, Martz E, Chay O, Mayrose I, Pupko T, et al. ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res. 2016;44:W344–50.
Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:1–7.
Zezos P, Patsiaoura K, Nakos A, Mpoumponaris A, Vassiliadis T, Giouleme O, et al. Severe eosinophilic infiltration in colonic biopsies predicts patients with ulcerative colitis not responding to medical therapy. Color Dis. 2014;16:O420–30.
Morgenstern S, Brook E, Rinawi F, Shamir R, Assa A. Tissue and peripheral eosinophilia as predictors for disease outcome in children with ulcerative colitis. Dig Liver Dis. 2017;49:170–4.
Bin Dhuban K, Piccirillo CA. The immunological and genetic basis of immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Curr Opin Allergy Clin Immunol. 2015;15:525–32.
Posovszky C. Congenital intestinal diarrheal diseases: a diagnostic and therapeutic challenge. Best Pract Res Clin Gastroenterol. 2016;30:187–211.
Caudy AA, Reddy ST, Chatila T, Atkinson JP, Verbsky JW. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J Allergy Clin Immunol. 2007;119:482–7.
Charbit-Henrion F, Jeverica AK, Bègue B, Markelj G, Parlato M, Avcin SL, et al. Deficiency in mucosa-associated lymphoid tissue lymphoma translocation 1: a novel cause of IPEX-like syndrome. J Pediatr Gastroenterol Nutr. 2017;64:378–84.
Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131:1611–1623.e3.
Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2013;4:6–11.
Alroqi FJ, Charbonnier LM, Keles S, Ghandour F, Mouawad P, Sabouneh R, et al. DOCK8 deficiency presenting as an IPEX-like disorder. J Clin Immunol. 2017;37:811–9.
Charbonnier LM, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J Allergy Clin Immunol. 2015;135:217–27.
Liang Y, Cucchetti M, Roncagalli R, Yokosuka T, Malzac A, Bertosio E, et al. The lymphoid lineage–specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat Immunol. 2013;14:858–66.
Matsushima N, Tachi N, Kuroki Y, Enkhbayar P, Osaki M, Kamiya M, et al. Structural analysis of leucine-rich-repeat variants in proteins associated with human diseases. Cell Mol Life Sci. 2005;62:2771–91.
Peters-Golden M, Henderson WR. Leukotrienes. N Engl J Med. 2007;357:1841–54.
Acknowledgments
We thank the patient and his family for participating in this study.
Funding
D.S.S. is supported by the Israel Science Foundation and Jeffrey Modell Foundation grants. S.B.S. is supported by NIH grants HL59561, DK034854, and AI50950; the Helmsley Charitable Trust; and the Wolpow Family Chair in IBD Treatment and Research.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
The study was approved by the institutional Helsinki committee, and written informed consent was obtained as customary.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 772 kb)
Rights and permissions
About this article
Cite this article
Kurolap, A., Eshach Adiv, O., Konnikova, L. et al. A Unique Presentation of Infantile-Onset Colitis and Eosinophilic Disease without Recurrent Infections Resulting from a Novel Homozygous CARMIL2 Variant. J Clin Immunol 39, 430–439 (2019). https://doi.org/10.1007/s10875-019-00631-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-019-00631-6