Abstract
Successful vaccines contain an adjuvant component that activates the innate immune system, thereby eliciting antigen-specific immune responses. Many adjuvants appear to be ligands for toll-like receptors (TLR), which are thus promising targets for the development of novel adjuvants to elicit vaccine immunogenicity. However, recent evidence suggests that some adjuvants activate the innate immune system in a TLR-independent manner possibly through other pattern recognition receptors and signaling machinery. In particular, newly identified intracellular retinoic-acid-inducible gene (RIG)-like receptors, NOD-like receptors, or even as yet unknown recognition machinery for the adjuvant may regulate TLR-independent vaccine immunogenicity. To develop optimal vaccines, it will be critical to understand how TLR-dependent and TLR-independent innate immune activation, by various adjuvants, control the consequent adaptive immune responses to vaccine.
Similar content being viewed by others
INTRODUCTION
The basic concept of a vaccine is to trigger the host immune system and mount adaptive immune responses of sufficient magnitude and duration, including B-cell-mediated antibody production and/or specific T-cell-mediated cellular responses to a protective antigen(s) in order to prevent infection or reduce the related pathology. It is now well-known that successful vaccines should contain not only such a protective antigen(s), but also a good adjuvant that efficiently activates the innate immune system for optimal vaccine immunogenicity.
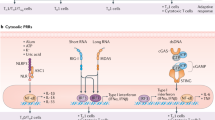
It has been shown that toll-like receptor (TLR), one of the innate immune sensors, plays important roles not only in the initial proinflammatory responses, but also in the consequent antigen-specific immune responses, both of which are crucial for protective immunity against infectious diseases (1–3). A variety of immunostimulatory compounds, including protein, lipid, carbohydrates, and nucleic acids, have been shown to be TLR ligands and are currently being used experimentally or in clinical trials within vaccine formulations as an adjuvant. However, recent evidence has shown that conventional adjuvants such as aluminium hydroxide (Alum), incomplete and complete Freund's adjuvant (IFA or CFA), or unconventional adjuvant-containing vehicle such as apoptotic cells and virus, elicit efficient adaptive immune responses to vaccine in the absence of TLRs (4–6). Moreover, newly characterized intracellular innate receptors that sense a variety of immunomodulatory compounds, such as NOD-like receptors (NLR), RIG-like receptors (RLR) and yet unknown intracellular DNA receptors, have been demonstrated to activate the innate immune responses, and possibly the adaptive immune responses, in a TLR-independent manner (7–9). Thus, it is important for us to understand how these innate sensors or their downstream signaling pathway(s) mediate the adjuvant-induced innate and adaptive immune responses in order to develop potent, but also safe, vaccine or related immunotherapy. Here, we review recent advances in our understanding of the TLR-dependent and TLR-independent adjuvant activity of vaccine components.
Specific Delivery and Targeting of an Adjuvant to the Cognate TLR for Vaccine Potency and Safety
Most TLR ligands, especially, those that can be chemically synthesized or genetically modified, are now under development as candidate vaccine adjuvants ((10, 11) and Table I). These TLR agonists are very potent adjuvants in capacity of activating cells expressing the cognate TLR, in particular, dendritic cells (DCs), which are the key antigen presenting cells. DCs produce cytokines, chemokines, and interferons, and up-regulate their functions, including antigen processing and presentation to naïve T cells. It has been shown that coadministration of vaccine (antigen) and TLR agonist, in the form of direct conjugation, or incorporation into an efficient targeting vehicle (a delivery system such as a viral particle, liposome, or an attached antibody against a surface molecule on DCs) into antigen presenting cells via the endosomal pathway, is necessary for the optimal vaccine formulation (12, 13).
When we consider utilizing TLR-agonists as adjuvants in vaccine development, it will be important to appreciate that the intracellular localization of TLRs is quite distinct between subfamilies. While certain TLRs (TLRs 1, 2, 4, 5, 6, and possibly, 10 and 11) are expressed on the cell surface, others (TLRs 3, 7, 8, and 9) are found almost exclusively in intracellular compartments such as the endoplasmic reticulum and endosomes. Cell surface TLR1, TLR2, and TLR6 recognize lipoproteins, TLR4 recognizes lipopolysaccharide (LPS), and TLR5 (or TLR11, which is not functional in humans though) recognizes a pathogen-derived protein; by contrast, endosomal TLR3 and TLR7, TLR8, and TLR9 recognize nucleic acids (14, 15). The physiological meaning of these distinct expression patterns between cell types and intracellular compartments is yet to be elucidated, but it is thought that ligands easily liberated from pathogens, such as flagellin, lipoprotein, and LPS on a pathogen's surface, are recognized by the host's cell surface TLRs, while ligands hidden inside the pathogens, such as nucleic acids, are recognized in endosomes after lysosomal degradation of microbes or cells. Such evidence can be translated into vaccine formulation and delivery systems. Not only vaccines need to target antigen presenting cells, but also the antigen and adjuvant need to reside in the same vesicle in a cell for efficient antigen processing and presentation through the endosomal or phagosomal pathway, coupled with TLR-dependent DC activation/maturation in order to prime CD4 T cells (16).
In addition, expression of each TLR is also quite distinct among cell types. TLR2 and 4 are expressed on various immune cells including macrophages, DCs, B cells, granulocytes, NK cells, and T cells, and even on nonimmune cells such as fibroblasts and epithelial cells. TLR7 and TLR9 are largely expressed in the immune cells. In particular, these receptors are predominantly expressed in plasmacytoid DCs that produce a large amount of type-I interferon during viral infection. The intercellular crosstalk between these TLR-expressing cells may influence the outcome of adjuvant-induced adaptive immune responses. In the case of viral, DNA, or RNA antigens, which are expressed inside cells, cross-presentation of antigen to CD8 T cells is known to occur, during which TLRs in nonantigen presenting cells may affect the outcome. TLR expression can be altered in response to a variety of cytokines and environmental stresses induced by pathogens or vaccines. Thus, the efficient and specific delivery of vaccine antigen as well as adjuvant into antigen processing and/or presenting cells should be carefully considered for potent, but also safe, vaccine development.
TLR2/4 and NOD1/2 as Sensors for a Bacterial Cell-Wall-Based Adjuvant
TLR2 and TLR4 on the cell surface, and intracellular proteins such as NOD1 and NOD2, which contain a nucleotide-binding oligomerization domain, are known to recognize distinct components within bacterial cell walls; these include LPS (recognized by TLR4), lipoprotein (recognized by TLR2), peptideglycan (PGN) (recognized by NOD) and lipoteichoic acid (LTA) (recognized by TLR2). It is known that the adjuvant activity of bacterial cell walls is responsible for their ability to activate the innate immune system through cognate receptor(s), and purified components of bacterial cell walls have also been proven to be potent adjuvants. The TLR4 ligand LPS has been experimentally shown to be a potent adjuvant for vaccines, although its extreme toxicity prevents its use in humans (17, 18). The adjuvant effect of LPS is solely dependent on TLR4-mediated, MyD88-dependent signaling (19, 20). Efforts to eliminate the toxicity of lipid A led to the development of monophosphoryl lipid A (MPL) (17, 18). MPL-based adjuvant (monophosphoryl-lipid A/trehalose dicorynomycolate (“Ribi” adjuvant)) has been used in human clinical studies as a new-generation vaccine adjuvant against infectious diseases and seasonal allergic rhinitis, and was proved to be safe and effective (21, 22). MPL contains lipid A as a TLR4 ligand; however, it was recently shown that the dependency of TLR4 on adjuvant effect of MPL was surprisingly minor, at least for antigen-specific antibody responses (4), suggesting that there are yet unknown TLR-independent adjuvant factors within the MPL compound.
TLR2 mediates the adjuvant activity of its ligand, lipoprotein; for example, Mycoplasma macrophage-activating lipopeptide 2 (MALP-2) is recognized by a heterodimer of TLR2 and TLR6, and the synthetic bacterial lipopeptide PAM3CSK4 is recognized by a dimer of TLR2 and TLR1 (23, 24), both of which have been proven to be potent adjuvants in vivo (25, 26). Outer-surface lipoprotein (OspA) of Borrelia burgdorferi, which is used in vaccines for Lyme disease, and conjugate polysaccharide vaccines containing outer membrane protein complex derived from Haemophilus influenzae type b (Hib-OMPC) are both potent vaccine formulations. OspA and Hib-OMPC not only contain protective antigen, but also contain immunostimulatory cell wall components as an adjuvant mainly recognized by TLR2. In humans, low responders to OspA vaccine have impaired expression of TLR1, and TLR1−/− as well as TLR2−/− mice were unable to mount a protective response after OspA vaccination (27). However, recent evidence suggests that there may be other adjuvant factors within the OspA vaccine formulation as TLR2−/− mice were protected by a Pam3Cys-modified OspA vaccine (28). Similarly, Hib-OMPC vaccine-induced proinflammatory cytokines were TLR2-dependent; however, antigen-specific IgG titers were not dramatically reduced in the absence of TLR2 (29), suggesting the existence of other adjuvant factors in this vaccine formulation.
In addition to the major role of TLR2 and 4 in the cell surface recognition of antigens and the subsequent activation of the innate immune system, NOD1 and NOD2, which are localized in the cytoplasm, have been shown to recognize PGN, a component of the bacterial cell wall (30, 31). Muramyldipeptide (MDP), a common structural component of PGN, is a ligand for NOD2, and other bioactive moieties of PGN, desmuramylpeptides (DMP) containing diaminopimelic acid (DAP), were found to be ligands for NOD1. Interestingly, MDP is a minimally required component of complete Freund's adjuvant (CFA), which is composed of a mycobacterial extract in an oil emulsion and is one of the most common adjuvants used experimentally (32). Although purified MDP is capable of inducing innate immune responses in human cells (but not in mouse cells) strong synergisms have been observed with the other TLR ligands (33, 34); these synergisms may contribute to the whole adjuvant activity of CFA, as CFA seems to contain TLR2 and/or TLR4 ligands. Similarly, the BCG vaccine, which is known to contain TLR2 and TLR4 ligands (as well as TLR9 ligand), has been shown to be able to induce adaptive immune responses in the absence of MyD88, a critical adaptor for TLR-mediated innate immune activations, suggesting that the BCG vaccine may contain TLR-independent adjuvant activity, probably NOD-like receptor ligands (35). Further studies should clarify which component(s) and host receptor(s) are critical for the adjuvant activity within these potent vaccine and adjuvant formulations.
TLR5 and NOD-Like Proteins Mediate the Flagellin-Induced Adjuvant Effect
TLR5 recognizes the bacterial protein flagellin, which is found in the flagellar structures of many bacteria (36). TLR5 is detected in epithelia in the lung and gut, and is also highly expressed in residual dendritic cells such as those in the lamina propria of the intestine (37). Flagellin is a potent immune activator, stimulating diverse biologic effects that mediate both innate inflammatory adaptive immune responses. The protein nature of flagellin is considered to be an advantage for many immuno-therapeutic applications mainly due to its ease of manipulation; for example, a DNA vaccine encoding a chimeric version of antigenic protein and flagellin has been developed (38, 39).
TLR5, however, appears not to be the only receptor that mediates the flagellin-induced adjuvant effect. Independently of TLR5 or MyD88, a member of the NOD-LRR protein family, neuronal apoptosis inhibitory protein 5 (NAIP5), has been shown to be involved in the detection of flagellin in the cytoplasm as well as in the caspase-1-dependent control of Legionella pneumophila infection by macrophages (40, 41). ICE protease activating factor (IPAF), another CARD-containing NOD-LRR protein, has been shown to recognize Salmonella typhimurium, whose infection also results in caspase-1 activation. Flagellin delivered to the cytosol activates caspase-1 via IPAF, and independently of TLR5 (42, 43). Although the mechanism by which these two proteins recognize the same ligand is not yet clear, NAIP5 and IPAF may cooperate in the recognition of such bacterial components as they can physically interact with each other. It will be of interest to clarify how the potent adjuvant activities of flagellin are mediated by these three flagellin receptors: cell-surface TLR5, the intracellular NOD-like protein NAIP5, and IPAF.
TLR3, 7, and 8, and RIG-Like Receptors Mediate the RNA-Induced Adjuvant Effect
TLR3 recognizes double-stranded (ds) RNA derived from the viral genome, or intermediates generated during viral replication, all of which have been shown to play an important role in antiviral responses. Poly-I:C, a synthetic version of dsRNA was one of the first therapeutic agents used to treat HIV and leukemia patients, but was abandoned due to its toxicity (44). Several studies have been undertaken to reduce the toxicity of poly-I:C, and this agent is currently undergoing clinical trials for breast cancer and ovarian cancer (45). Importantly, the dsRNA-induced, TLR3-mediated maturation of CD8 dendritic cells was shown to play an important role in the induction of antigen-specific CD4+ and CD8+ T cell responses via type I interferon-mediated cross-priming, suggesting that TLR3 is a good adjuvant target for inducing cellular immune responses (46, 47). However, dsRNA still stimulated dendritic cells in TLR3−/− mice, especially, when administered directly into the cytosol by transfection, indicating the existence of a TLR3-independent adjuvant receptor for dsRNA.
Recently, three homologous DExD/H box RNA helicases were identified as cytoplasmic sensors for viral infection and dsRNA (48, 49). Two family members, retinoic-acid-inducible gene I (RIG-I) (also called DDX58) and melanoma-differentiation-associated gene 5 (MDA5) (also called Helicard), share two N-terminal CARDs followed by an RNA helicase domain (48). RIG-I and MDA5 differentially sense invasion of a variety of RNA viruses by recognizing distinct features of the RNA genome or RNA products and trigger a TLR-independent signaling pathway through IPS-1, culminating in antiviral immune responses including type-I IFN production (50). Surprisingly, recent evidence suggests that MDA5, but not RIG-I, is essential for TLR3-independent, Poly-I:C-mediated innate immune responses, including type-I IFN production and dendritic cell activation (51, 52). These results also provide the important information that MDA5 may play a role not only in innate antiviral responses, but also in adaptive immune responses to virus or vaccine where dsRNA acts as an adjuvant.
Unlike dsRNA, single-stranded RNA had long been thought to be immunologically inert, because host cells are abundant with single-stranded RNA species. However, recent evidence suggests that single-stranded RNA is not inert, but rather very immunostimulatory unless heavily modified by methylation or with certain sequences (53–56) that may explain the ability of single-stranded RNA to reach endosomes (57). Single-stranded RNA genomes, oligoribonucleotides derived from HIV or influenza virus, some double-stranded short interference (si) RNAs developed for RNA interference (RNAi), and small synthetic compounds known as imidazoquinolins, are recognized by TLR7 in mice, and by both TLR7 and TLR8 in humans; this recognition activates various immune cells that produce type I IFNs and elicit cellular immune responses (58–60). In humans, TLR7, but not TLR8, is highly expressed in plasmacytoid DCs, and activation of TLR7 in these cells leads to the production of type-I IFNs. By contrast, TLR8 (but not TLR7) is highly expressed in monocytes, and activation of TLR8 in these cells leads to the production of proinflammatory cytokines, especially, IL-12 (61). TLR7 and possibly TLR8 utilize MyD88 as an essential adaptor to downstream signaling pathways. Several TLR7 agonists have been approved for clinical use in various viral infections (62). The TLR7 agonist imiquimod (5% cream) has been shown to be effective for external genital warts, basal cell carcinoma, and actinic keratosis (63–65), and is in a phase I clinical trial against human papillomavirus (22). Several other synthetic TLR7 agonist compounds have been in phase I or phase II trials against hepatitis B virus, hepatitis C virus, and cancer (22). The adjuvant activity of TLR7 ligand was also confirmed in nonhuman primates (66).
However, immunostimulatory single-stranded RNA derived from either RNA viruses, such as influenza, or synthetic oligoribonucleotides has been reported to stimulate the immune system in a TLR7/8-independent manner also. While immunostimulatory RNA and the RNA genomes of viruses such as influenza activate plasmacytoid DCs via TLR7, they were also able to activate myeloid cells, such as monocytes, conventional DCs, or fibroblasts, in a TLR7- or MyD88-independent manner (6, 55, 67). Moreover, recent studies suggest that RIG-I, in fact, recognizes 5′-triphosphate of single-stranded RNA (68, 69). Thus, it is important for us to know which innate immune receptors, TLRs and/or RIG-like receptors are critical for the induction of protective innate and adaptive immune responses during viral infection or vaccination with an RNA-based vaccine that may contain immunostimulatory RNA as an internal adjuvant. By knowing these details, we will be able to efficiently target such an RNA-containing vaccine to the right cells, and optimize their adjuvant activity depending on the innate immune receptors described above, to provide protective immune responses.
TLR9-Dependent and TLR9-Independent Adjuvant Effect of DNA
As a fundamental entity of most living organisms, DNA is normally tightly sequestered within the nuclear or mitochondrial membranes in eukaryotes, the cell wall in bacteria, or the envelope in viruses. However, in the circumstances of microbial infection or failure of host DNA clearance, DNA can be released from microbes or damaged host cells, and is detected by and modulates the innate immune system. Currently, TLR9, the only known receptor to detect immunostimulatory DNA such as CpG DNA, has been shown to play critical roles in mediating the protective immune responses to various infectious agents, allergic disorders, and cancer, and is implicated in a pathological role in certain autoimmune diseases (reviewed in (70–72)). Synthetic oligodeoxynucleotides (ODNs) that contain unmethylated CpG motifs trigger TLR9-mediated, MyD88-dependent signaling in macrophages, dendritic cells, and B cells to induce the production of proinflammatory cytokines, chemokines, and immunoglobulins. The robust innate immune response to CpG ODNs skews the host's immune milieu in favor of a strong cellular immune response, including induction of CD4 Th1 and CD8 CTL, an effect that underlies their use as vaccine adjuvants and anti-allergens. Preclinical studies provide evidence that CpG ODNs are effective for each of these uses and can modulate the immune response to coadministered allergens and vaccines (73, 74).
Plasmid DNA derived from bacteria contains immunostimulatory CpG motifs (72), which have been shown to stimulate the innate immune system; thus, these motifs can act as a “built-in” adjuvant for DNA vaccines (75). TLR9 is currently the only known receptor for the immunostimulatory CpG motifs in DNA, and TLR9-deficient antigen presenting cells, including dendritic cells, do not respond to CpG motifs (76). As expected, TLR9-deficient mice failed to mount Th1-biased antigen-specific immune responses to protein vaccines using CpG ODN as an adjuvant (76).
However, in the case of DNA vaccines, TLR9-deficient mice mounted a comparable amount of the encoded-antigen-specific IgG, including IgG1 and IgG2a, IFNγ secretion and CTL responses, to the amounts produced by wild-type mice (77, 78); another report showed a partial reduction of immune responses in TLR9-deficient mice (79). Moreover, recent evidence suggests that not only DNA derived from microbes, but also DNA derived from host cells, activates the innate immune system in a CpG motif-independent manner that is dependent on its double-stranded (ds) structure when it is introduced into the cytosol (80, 81) or if the homeostatic clearance of such DNA is hampered, this pathway is activated (82). Double-stranded (ds) DNA in the right-handed B-form (B-DNA), but to a lesser extent in the left-handed Z-form (Z-DNA), activates both immune and nonimmune cells to produce type I interferons (IFNs), cytokines, and chemokines through a TLR9-independent pathway, but as an yet undefined DNA recognition machinery, and a distinct signaling pathway in which TBK1, a noncanonical IkB kinase is involved (83, 84). These results suggest that the immunogenicity of DNA vaccines is controlled mainly by TLR9-independent and, possibly, CpG-motif-independent factors in the plasmid DNA that act as “built-in” adjuvants. It will be of interest to investigate whether TLR9-independent innate immune recognition of and regulation by DNA provide clues to the understanding of their physiological roles in the immunogenicity of DNA-based vaccines or immunotherapy.
Concluding Remarks
As TLR-related research on the innate immune system matures, TLR-independent pathway(s), which control not only innate, but also adaptive immune responses, have emerged. Further understanding of both pathways of innate immune recognition and regulation by many immunologically active compounds will hopefully facilitate the development of more potent and safer adjuvants, ultimately toward protective vaccines for applicable diseases.
References
Janeway CA, Jr., Medzhitov R: Innate immune recognition. Annu Rev Immunol. 20:197–216, 2002
Akira S, Uematsu S, Takeuchi O: Pathogen recognition and innate immunity. Cell 124:783–801, 2006
Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K: Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu Rev Immunol 24:353–389, 2006
Gavin AL, Hoebe K, Duong B, Ota T, Martin C, Beutler B, Nemazee D: Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science 314:1936–1938, 2006
Janssen E, Tabeta K, Barnes MJ, Rutschmann S, McBride S, Bahjat KS, Schoenberger SP, Theofilopoulos AN, Beutler B, Hoebe K: Efficient T cell activation via a Toll-Interleukin 1 receptor-independent pathway. Immunity 24:787–799, 2006
Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM: TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol 173:6882–6889, 2004
Creagh EM, O’Neill LA: TLRs, NLRs and RLRs: A trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 27:352–357, 2006
Meylan E, Tschopp J, Karin M: Intracellular pattern recognition receptors in the host response. Nature 442:39–44, 2006
Ishii KJ, Akira S: Innate immune recognition of, and regulation by, DNA. Trends Immunol 27:525–532, 2006
Kaisho T, Akira S: Toll-like receptors as adjuvant receptors. Biochim Biophys Acta 1589:1–13, 2002
van Duin D, Medzhitov R, Shaw AC: Triggering TLR signaling in vaccination. Trends Immunol 27:49–55, 2006
O’Hagan DT, Valiante NM: Recent advances in the discovery and delivery of vaccine adjuvants. Nat Rev Drug Discov 2:727–735, 2003
Pashine A, Valiante NM, Ulmer JB: Targeting the innate immune response with improved vaccine adjuvants. Nat Med 11:S63–S68, 2005
Ishii KJ, Coban C, Akira S: Manifold mechanisms of toll-like receptor-ligand recognition. J Clin Immunol 25:511–521, 2005
Ishii KJ, Akira S: Innate immune recognition of nucleic acids: Beyond toll-like receptors. Int J Cancer 117:517–523, 2005
Blander JM, Medzhitov R: On regulation of phagosome maturation and antigen presentation. Nat Immunol 7:1029–1035, 2006
Masihi KN, Lange W, Brehmer W, Ribi E: Immunobiological activities of nontoxic lipid A: Enhancement of nonspecific resistance in combination with trehalose dimycolate against viral infection and adjuvant effects. Int J Immunopharmacol 8:339–345, 1986
Cluff CW, Baldridge JR, Stover AG, Evans JT, Johnson DA, Lacy MJ, Clawson VG, Yorgensen VM, Johnson CL, Livesay MT, Hershberg RM, Persing DH: Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect Immun 73:3044–3052, 2005
Pasare C, Medzhitov R: Toll-dependent control mechanisms of CD4 T cell activation. Immunity 21:733–741, 2004
Pasare C, Medzhitov R: Control of B-cell responses by toll-like receptors. Nature 438:364–368, 2005
Evans JT, Cluff CW, Johnson DA, Lacy MJ, Persing DH, Baldridge JR: Enhancement of antigen-specific immunity via the TLR4 ligands MPL adjuvant and Ribi. 529. Expert Rev Vaccines 2:219–229, 2003
Hoffman ES, Smith RE, Renaud RC, Jr.: From the analyst's couch: TLR-targeted therapeutics. Nat Rev Drug Discov 4:879–880, 2005
Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, Zychlinsky A, Takeda K, Akira S: Discrimination of bacterial lipoproteins by toll-like receptor 6. Int Immunol 13:933–940, 2001
Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, Dong Z, Modlin RL, Akira S: Cutting edge: Role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169:10–14, 2002
Patel M, Xu D, Kewin P, Choo-Kang B, McSharry C, Thomson NC, Liew FY: TLR2 agonist ameliorates established allergic airway inflammation by promoting Th1 response and not via regulatory T cells. J Immunol 174:7558–7563, 2005
Borsutzky S, Kretschmer K, Becker PD, Muhlradt PF, Kirschning CJ, Weiss S, Guzman CA: The mucosal adjuvant macrophage-activating lipopeptide-2 directly stimulates B lymphocytes via the TLR2 without the need of accessory cells. J Immunol 174:6308–6313, 2005
Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, Medzhitov R, Fikrig E, Flavell RA: Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in. Nat Med 8:878–884, 2002
Yoder A, Wang X, Ma Y, Philipp MT, Heilbrun M, Weis JH, Kirschning CJ, Wooten RM, Weis JJ: Tripalmitoyl-S-glyceryl-cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect Immun 71:3894–3900, 2003
Latz E, Franko J, Golenbock DT, Schreiber JR: Haemophilus influenzae type b-outer membrane protein complex glycoconjugate vaccine induces cytokine production by engaging human toll-like receptor 2 (TLR2) and requires the presence of TLR2 for optimal immunogenicity. J Immunol 172:2431–2438, 2004
Inohara N, Chamaillard M, McDonald C, Nunez G: NOD-LRR proteins: Role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355–383, 2005
Fritz JH, Ferrero RL, Philpott DJ, Girardin SE: NOD-like proteins in immunity, inflammation and disease. Nat Immunol 7:1250–1257, 2006
Ellouz F, Adam A, Ciorbaru R, Lederer E: Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun 59:1317–1325, 1974
Kufer TA, Sansonetti PJ: Sensing of bacteria: NOD a lonely job. Curr Opin Microbiol 2006
Tada H, Aiba S, Shibata K, Ohteki T, Takada H: Synergistic effect of NOD1 and NOD2 agonists with toll-like receptor agonists on human dendritic cells to generate interleukin-12 and T helper type 1 cells. Infect Immun 73:7967–7976, 2005
Fremond CM, Yeremeev V, Nicolle DM, Jacobs M, Quesniaux VF, Ryffel B: Fatal mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88. J Clin Invest 114:1790–1799, 2004
Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, Eng JK, Akira S, Underhill DM, Aderem A: The innate immune response to bacterial flagellin is mediated by toll-like receptor 5. Nature 410:1099–1103, 2001
Uematsu S, Jang MH, Chevrier N, Guo Z, Kumagai Y, Yamamoto M, Kato H, Sougawa N, Matsui H, Kuwata H, Hemmi H, Coban C, Kawai T, Ishii KJ, Takeuchi O, Miyasaka M, Takeda K, Akira S: Detection of pathogenic intestinal bacteria by toll-like receptor 5 on intestinal CD11c+ lamina propria cells. Nat Immunol 7:868–874, 2006
Applequist SE, Rollman E, Wareing MD, Liden M, Rozell B, Hinkula J, Ljunggren HG: Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol 175:3882–3891, 2005
Honko AN, Sriranganathan N, Lees CJ, Mizel SB: Flagellin is an effective adjuvant for immunization against lethal respiratory challenge with Yersinia pestis. Infect Immun 74:1113–1120, 2006
Molofsky AB, Byrne BG, Whitfield NN, Madigan CA, Fuse ET, Tateda K, Swanson MS: Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203:1093–1104, 2006
Zamboni DS, Kobayashi KS, Kohlsdorf T, Ogura Y, Long EM, Vance RE, Kuida K, Mariathasan S, Dixit VM, Flavell RA, Dietrich WF, Roy CR: The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7:318–325, 2006
Franchi L, Amer A, Body-Malapel M, Kanneganti TD, Ozoren N, Jagirdar R, Inohara N, Vandenabeele P, Bertin J, Coyle A, Grant EP, Nunez G: Cytosolic flagellin requires Ipaf for activation of caspase-1 and interleukin 1beta in salmonella-infected macrophages. Nat Immunol 7:576–582, 2006
Miao EA, Alpuche-Aranda CM, Dors M, Clark AE, Bader MW, Miller SI, Aderem A: Cytoplasmic flagellin activates caspase-1 and secretion of interleukin 1beta via Ipaf. Nat Immunol 7:569–575, 2006
Robinson RA, DeVita VT, Levy HB, Baron S, Hubbard SP, Levine AS: A phase I-II trial of multiple-dose polyriboinosic-polyribocytidylic acid in patieonts with leukemia or solid tumors. J Natl Cancer Inst 57:599–602, 1976
Adams M, Navabi H, Jasani B, Man S, Fiander A, Evans AS, Donninger C, Mason M: Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R). Vaccine 21:787–790, 2003
Schulz O, Diebold SS, Chen M, Naslund TI, Nolte MA, Alexopoulou L, Azuma YT, Flavell RA, Liljestrom P, Reis e Sousa C: Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887–892, 2005
Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, Bosio C, Dow S: Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol 176:7335–7345, 2006
Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T: The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol 5:730–737, 2004
Yoneyama M, Kikuchi M, Matsumoto K, Imaizumi T, Miyagishi M, Taira K, Foy E, Loo YM, Gale M, Jr., Akira S, Yonehara S, Kato A, Fujita T: Shared and unique functions of the DExD/H-Box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J Immunol 175:2851–2858, 2005
Kawai T, Akira S: Innate immune recognition of viral infection. Nat Immunol 7:131–137, 2006
Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S: Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105, 2006
Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M: Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci USA 103:8459–8464, 2006
Kariko K, Bhuyan P, Capodici J, Weissman D: Small interfering RNAs mediate sequence-independent gene suppression and induce immune activation by signaling through toll-like receptor 3. J Immunol 172:6545–6549, 2004
Ishii KJ, Akira S: TLR ignores methylated RNA? Immunity 23:111–113, 2005
Sugiyama T, Gursel M, Takeshita F, Coban C, Conover J, Kaisho T, Akira S, Klinman DM, Ishii KJ: CpG RNA: Identification of novel single-stranded RNA that stimulates human CD14+CD11c+ monocytes. J Immunol 174:2273–2279, 2005
Koski GK, Kariko K, Xu S, Weissman D, Cohen PA, Czerniecki BJ: Cutting edge: Innate immune system discriminates between RNA containing bacterial versus eukaryotic structural features that prime for high-level IL-12 secretion by dendritic cells. J Immunol 172:3989–3993, 2004
Diebold SS, Massacrier C, Akira S, Paturel C, Morel Y, Reis e Sousa C: Nucleic acid agonists for toll-like receptor 7 are defined by the presence of uridine ribonucleotides. Eur J Immunol 36:3256–3267, 2006
Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K, Akira S: Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol 3:196–200, 2002
Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C: Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529–1531, 2004
Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, Lipford G, Wagner H, Bauer S: Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526–1529, 2004
Gorden KB, Gorski KS, Gibson SJ, Kedl RM, Kieper WC, Qiu X, Tomai MA, Alkan SS, Vasilakos JP: Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol 174:1259–1268, 2005
Horsmans Y, Berg T, Desager JP, Mueller T, Schott E, Fletcher SP, Steffy KR, Bauman LA, Kerr BM, Averett DR: Isatoribine, an agonist of TLR7, reduces plasma virus concentration in chronic hepatitis C infection. Hepatology 42:724–731, 2005
McInturff JE, Modlin RL, Kim J: The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol 125:1–8, 2005
Stockfleth E, Trefzer U, Garcia-Bartels C, Wegner T, Schmook T, Sterry W: The use of toll-like receptor-7 agonist in the treatment of basal cell carcinoma: An overview. Br J Dermatol 149(Suppl 66):53–56, 2003
Lysa B, Tartler U, Wolf R, Arenberger P, Benninghoff B, Ruzicka T, Hengge UR, Walz M: Gene expression in actinic keratoses: Pharmacological modulation by imiquimod. Br J Dermatol 151:1150–1159, 2004
Wille-Reece U, Flynn BJ, Lore K, Koup RA, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA: HIV Gag protein conjugated to a toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc Natl Acad Sci USA 102:15190–15194, 2005
Kato H, Sato S, Yoneyama M, Yamamoto M, Uematsu S, Matsui K, Tsujimura T, Takeda K, Fujita T, Takeuchi O, Akira S: Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19–28, 2005
Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G: 5′-Triphosphate RNA is the ligand for RIG-I. Science 314:994–997, 2006
Pichlmair A, Schulz O, Tan CP, Naslund TI, Liljestrom P, Weber F, Reis e Sousa C: RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314:997–1001, 2006
Klinman DM: Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol 4:249–258, 2004
Wagner H: The immunobiology of the TLR9 subfamily. Trends Immunol 25:381–386, 2004
Krieg AM: Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discov 5:471–484, 2006
Broide DH: Immunostimulatory sequences of DNA and conjugates in the treatment of allergic rhinitis. Curr Allergy Asthma Rep 5:182–185, 2005
Vollmer J: TLR9 in health and disease. Int Rev Immunol 25:155–181, 2006
Gurunathan S, Klinman DM, Seder RA: DNA vaccines: Immunology, application, and optimization*. Annu Rev Immunol 18:927–974, 2000
Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, Akira S: A toll-like receptor recognizes bacterial DNA. Nature 408:740–745, 2000
Spies B, Hochrein H, Vabulas M, Huster K, Busch DH, Schmitz F, Heit A, Wagner H: Vaccination with plasmid DNA activates dendritic cells via toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J Immunol 171:5908–5912, 2003
Babiuk S, Mookherjee N, Pontarollo R, Griebel P, van Drunen Littel-van den Hurk S, Hecker R, Babiuk L: TLR9−/− and TLR9+/+ mice display similar immune responses to a DNA vaccine. Immunology 113:114–120, 2004
Tudor D, Dubuquoy C, Gaboriau V, Lefevre F, Charley B, Riffault S: TLR9 pathway is involved in adjuvant effects of plasmid DNA-based vaccines. Vaccine 23:1258–1264, 2005
Suzuki K, Mori A, Ishii KJ, Saito J, Singer DS, Klinman DM, Krause PR, Kohn LD: Activation of target-tissue immune-recognition molecules by double-stranded polynucleotides. Proc Natl Acad Sci USA 96:2285–2290, 1999
Ishii KJ, Suzuki K, Coban C, Takeshita F, Itoh Y, Matoba H, Kohn LD, Klinman DM: Genomic DNA released by dying cells induces the maturation of APCs. J Immunol 167:2602–2607, 2001
Kawane K, Fukuyama H, Yoshida H, Nagase H, Ohsawa Y, Uchiyama Y, Okada K, Iida T, Nagata S: Impaired thymic development in mouse embryos deficient in apoptotic DNA degradation. Nat Immunol 4:138–144, 2003
Ishii KJ, Coban C, Kato H, Takahashi K, Torii Y, Takeshita F, Ludwig H, Sutter G, Suzuki K, Hemmi H, Sato S, Yamamoto M, Uematsu S, Kawai T, Takeuchi O, Akira S: A toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat Immunol 7:40–48, 2006
Stetson DB, Medzhitov R: Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93–103, 2006
Spohn R, Buwitt-Beckmann U, Brock R, Jung G, Ulmer AJ, Wiesmuller KH: Synthetic lipopeptide adjuvants and toll-like receptor 2—structure-activity relationships. Vaccine 22:2494–2499, 2004
Rharbaoui F, Drabner B, Borsutzky S, Winckler U, Morr M, Ensoli B, Muhlradt PF, Guzman CA: The Mycoplasma-derived lipopeptide MALP-2 is a potent mucosal adjuvant. Eur J Immunol 32:2857–2865, 2002
Becker PD, Bertot GM, Souss D, Ebensen T, Guzman CA, Grinstein S: Intranasal vaccination with recombinant CD protein and adamantylamide dipeptide as mucosal adjuvant enhances pulmonary clearance of Moraxella catarrhalis in a murine experimental model. Infect Immun 2006
Alexopoulou L, Holt AC, Medzhitov R, Flavell RA: Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature 413:732–738, 2001
Ren T, Zamboni DS, Roy CR, Dietrich WF, Vance RE: Flagellin-deficient Legionella mutants evade caspase-1—and Naip5-mediated macrophage immunity. PLoS Pathog 2:e18, 2006
Wille-Reece U, Flynn BJ, Lore K, Koup RA, Miles AP, Saul A, Kedl RM, Mattapallil JJ, Weiss WR, Roederer M, Seder RA: Toll-like receptor agonists influence the magnitude and quality of memory T cell responses after prime-boost immunization in nonhuman primates. J Exp Med 203:1249–1258, 2006
Westwood A, Elvin SJ, Healey GD, Williamson ED, Eyles JE: Immunological responses after immunisation of mice with microparticles containing antigen and single stranded RNA (polyuridylic acid). Vaccine 24:1736–1743, 2006
Coban C, Ishii KJ, Sullivan DJ, Kumar N: Purified malaria pigment (hemozoin) enhances dendritic cell maturation and modulates the isotype of antibodies induced by a DNA vaccine. Infect Immun 70:3939–3943, 2002
Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S: Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med 201:19–25, 2005
Fleck J, Mock M, Tytgat F, Nauciel C, Minck R: Adjuvant activity in delayed hypersensitivity of the peptidic part of bacterial peptidoglycans. Nature 250:517–518, 1974
Takada H, Uehara A: Enhancement of TLR-mediated innate immune responses by peptidoglycans through NOD signaling. Curr Pharm Des 12:4163–4172, 2006
Martinon F, Agostini L, Meylan E, Tschopp J: Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol 14:1929–1934, 2004
Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G: Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233–236, 2006
Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM: Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440:228–232, 2006
Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J: Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440:237–241, 2006
Gupta RK: Aluminum compounds as vaccine adjuvants. Adv Drug Deliv Rev 32:155–172, 1998
Aucouturier J, Ascarateil S, Dupuis L: The use of oil adjuvants in therapeutic vaccines. Vaccine 24(Suppl 2):S2–S5, 2006
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ishii, K.J., Akira, S. Toll or Toll-Free Adjuvant Path Toward the Optimal Vaccine Development. J Clin Immunol 27, 363–371 (2007). https://doi.org/10.1007/s10875-007-9087-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10875-007-9087-x
KEY WORDS:
- Innate immunity
- adaptive immunity
- toll-like receptor (TLR)
- vaccine
- adjuvant
- dendritic cells
- monophosphoryl lipid A (MPL)
- outer-surface lipoprotein (OspA)
- Hib-OMPC
- NOD
- desmuramylpeptides (DMP)
- muramyldipeptide (MDP)
- complete Freund's adjuvant (CFA)
- incomplete Freund's adjuvant (IFA)
- bacille calmette guerin (BCG)
- flagellin
- DNA vaccine
- ICE protease activating factor (IPAF)
- neuronal apoptosis inhibitory protein 5 (NAIP5)
- dsRNA
- Poly-I:C
- retinoic-acid-inducible gene I (RIG-I)
- melanoma-differentiation-associated gene 5 (MDA5)
- IPS-1
- ssRNA
- interferon
- B-DNA
- Z-DNA
- CpG DNA