Abstract
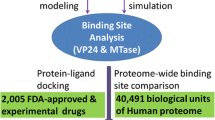
The Epstein-Barr Nuclear Antigen 1 (EBNA1) is a critical protein encoded by the Epstein-Barr Virus (EBV). During latent infection, EBNA1 is essential for DNA replication and transcription initiation of viral and cellular genes and is necessary to immortalize primary B-lymphocytes. Nonetheless, the concept of EBNA1 as drug target is novel. Two EBNA1 crystal structures are publicly available and the first small-molecule EBNA1 inhibitors were recently discovered. However, no systematic studies have been reported on the structural details of EBNA1 “druggable” binding sites. We conducted computational identification and structural characterization of EBNA1 binding pockets, likely to accommodate ligand molecules (i.e. “druggable” binding sites). Then, we validated our predictions by docking against a set of compounds previously tested in vitro for EBNA1 inhibition (PubChem AID-2381). Finally, we supported assessments of pocket druggability by performing induced fit docking and molecular dynamics simulations paired with binding affinity predictions by Molecular Mechanics Generalized Born Surface Area calculations for a number of hits belonging to druggable binding sites. Our results establish EBNA1 as a target for drug discovery, and provide the computational evidence that active AID-2381 hits disrupt EBNA1:DNA binding upon interacting at individual sites. Lastly, structural properties of top scoring hits are proposed to support the rational design of the next generation of EBNA1 inhibitors.







Similar content being viewed by others
Abbreviations
- CD:
-
Core domain
- EBNA1:
-
Epstein-Barr nuclear antigen 1
- EBV:
-
Epstein Barr virus
- EC:
-
Extended chain
- FBDD:
-
Fragment-based drug design
- FD:
-
Flanking domain
- FP:
-
Fluorescence polarization
- HTS:
-
High-throughput screening
- MLS:
-
Minimum ligand scaffold
- PR:
-
Proline rich
- SBDD:
-
Structure-based drug design
- vHTS:
-
Virtual high-throughput screening
- VS:
-
Virtual screening
References
Young LS, Rickinson AB (2004) Nat Rev Cancer 4:757–768
Linder SE, Sugden B (2007) Plasmid 58:1–12
Kempkes B, Pich D, Zeidler R, Hammerschmidt W (1995) Proc Natl Acad Sci USA 92:5875–5879
Humme S, Reisbach G, Feederle R, Delecluse HJ, Bousset K, Hammerschmidt W, Schepers A (2003) Proc Natl Acad Sci USA 100:10989–10994
Bochkarev A, Barwell JA, Pfuetzner RA, Furey W Jr, Edwards AM, Frappier L (1995) Cell 83:39–46
Bochkarev A, Bochareva E, Frappier L, Edwards AM (1998) J Mol Biol 284:1273–1278
Cruickshank J, Shire K, Davidson AR, Edwards AM, Frappier L (2000) J Biol Chem 275:22273–22277
Ambinder RF, Shah WA, Rawlins DR, Hayward GS, Hayward SD (1990) J Virol 64:2369–2379
The Research Collaboratory for Structural Bioinformatics Protein Data Bank, RCSB PDB; http://www.rcsb.org/pdb. Accessed on 22 May 2013
Li N, Thompson S, Schults DC, Zhu W, Jiang H, Luo C, Lieberman PM (2010) PLoSONE (5)4:e10126
Thompson S, Messick T, Schultz DC, Reichman M, Lieberman PM (2011) J Biomol Screen 15:1107–1115
Kang MS, Lee EWK, Soni V, Lewis TA, Koehler AN, Srinivasan V, Kieff E (2011) J Virol 85:2859–2868
Duellman S, Thompson KL, Coon JJ, Burgess RR (2009) J Gen Virol 90:2251–2259
Yasuda A, Noguchi K, Minoshima M, Kashivazaki G, Kanda T, Katayama K, Mitsuhashi J, Bando T, Sugiyama H, Sugimoto Y (2011) Cancer Sci 102:2221–2230
Hopkins AL, Groom CR (2002) Nat Rev Drug Discov 1:727–730
National Center for Biotechnology Information. PubChem BioAssay Database; AID = 2381, Source = Scripps Research Institute Molecular Screening Center, Assay Provider: Paul Lieberman, Wistar Institute, http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=2381. Accessed 27 May 2013
Schrödinger Suite 2010 Protein Preparation Wizard; Epik version 2.1, Schrödinger, LLC, New York, NY, 2010; Impact, version 5.6, Schroödinger, LLC, New York, NY, 2005; Prime, version 2.2, Schroödinger, LLC, New York, NY
Jorgensen WJ, Maxwell DS, Tirado-Rives J (1996) J Am Chem Soc 118:11225–11236
Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL (2001) J Phys Chem B 105:6474–6487
Shivakumar D, Williams J, Wu J, Damn W, Shelly J, Sherman W (2010) J Chem Theory Comput 6:1509–1519
Site Finder: Molecular Operating Environment (MOE), 2012.10; Chemical Computing Group Inc., 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7, 2012
SiteMap, version 2.5, Schrödinger, LLC, New York, NY, 2011
LigPrep, version 2.5, Schrödinger, LLC, New York, 2012
Epik, version 2.2, Schrödinger, LLC, New York, 2012
Glide, version 5.7, Schrödinger, LLC, New York, NY, 2011
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelly M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) J Med Chem 47:1739–1749
Halgren TA, Murphy RB, Friesner RA, Behard HS, Frye LL, Pollard WT, Banks JL (2004) J Med Chem 47:1750–1759
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) J Med Chem 49:6177–6196
Lyne PD, Lamb ML, Saeh JC (2006) J Med Chem 49:4805–4808
Gianti E, Zauhar RJ (2015) J Comput Aided Mol Des 29:451–470
Sirin S, Kumar R, Martinez C, Karmilowicz MJ, Ghosh P, Abramov YA, Martin V, Sherman W (2014) J Chem Inf Model 54:2334–2346
Desmond Molecular Dynamics System, version 3.8, D. E. Shaw Research, New York, NY, 2014. Maestro-Desmond Interoperability Tools, version 3.8, Schrödinger, New York, NY, 2014
Jorgensen WL, Chandrasekhar J, Madura JD, Impey RW, Klein ML (1983) J Chem Phys 79:926–935
Humphrey W, Dalke A, Schulten K (1996) J Molec Graphics 14:33–38
Schultes S, de Graaf C, Haaksma EEJ, de Esch IJP, Leurs R, Krämer O (2010) Drug Discov Today Technol 7:e157–e162
Sherman W, Day T, Jacobson MP, Friesner RA, Farid R (2006) J Med Chem 49:534–553
Schrödinger Suite 2014-2 Induced Fit Docking protocol; Glide version 6.3, Schrödinger, LLC, New York, NY, 2014; Prime version 3.6, Schrödinger, LLC, New York, NY, 2014
Gianti E, Zauhar RJ (2012) J Chem Inf Model 52:2670–2683
Han SJ, Hu J, Pierce B, Weng Z, Renne R (2010) J Gen Virol 91:2203–2215
Ceccarelli DF, Frappier L (2000) J Virol 74:4939–4948
Labute P, Santavy M. Chemical Computing Group Inc. http://www.chemcomp.com/journal/sitefind.htm
Edelsbrunner H, Facello M, Fu R, Liang J (1995) Proceedings of the 28th annual Hawaii international conference on systems science, pp 256–264
Liang J, Edelsbrunner H, Woodward C (1998) Protein Sci 7:1884–1897
Clarkson KL (1992) Proceedings of 31st annual IEEE symposium on foundations of computer science, pp 387–395
Goodford PJ (1985) J Med Chem 28:849–857
Halgren T (2009) J Chem Inf Model 49:377–389
Halgren T (2007) Chem Biol Drug Des 69:146–148
Hetény C, Van Der Spoel C (2006) FEBS Lett 580:1447–1450
Hetény C, Van Der Spoel C (2002) Protein Sci 11:1729–1737
Huang N, Jacobson MP (2010) PLoS ONE 5(4):e10109
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (2001) Adv Drug Del Rev 46:3–26
Congreve M, Carr R, Murray C, Jhoti H (2003) Drug Discov Today 8:876–877
Valkov E, Sharpe T, Marsh M, Greive S, Hyvönen M (2012) Top Curr Chem 317:145–179
National Center for Biotechnology Information. PubChem BioAssay Database; AID = 2292, Source = Scripps Research Institute Molecular Screening Center, Assay Provider: Paul Lieberman, Wistar Institute, http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=2292. Accessed on 27 May 2013
National Center for Biotechnology Information. PubChem BioAssay Database; AID = 2338, Source = Scripps Research Institute Molecular Screening Center, Assay Provider: Paul Lieberman, Wistar Institute, http://pubchem.ncbi.nlm.nih.gov/assay/assay.cgi?aid=2338. Accessed on 27 May 2013
Huang SY, Zou X (2007) Proteins 66:399–421
Lewell XQ, Judd DB, Watson SP, Hann MM (1998) J Chem Inf Comput Sci 38:511–522
Gianti E, Sartori L (2008) J Chem Inf Model 48:2129–2139
Zauhar RZ, Moyna G, Tian L-F, Li Z-W, Welsh WJ (2003) J Med Chem 46:5674–5690
Zauhar RJ, Gianti E, Welsh WJ (2013) J Comput Aided Mol Des 12:1009–1036
Irwin JJ, Shoichet BK (2005) J Chem Inf Model 45:177–182
Irwin JJ, Sterling T, Mysinger MM, Bolstad ES, Coleman RG (2012) J Chem Inf Model 52:1757–1768
Grove LE, Hall DR, Beglov D, Vajda S, Kozakov D (2013) Bioinformatics 29:1218–1219
Adasme-Carreño F, Muñoz-Gutierrez C, Caballero J, Alzate-Morales JH (2014) Phys Chem Chem Phys 16:14047–14058
Messick TE (2015) Cambridge Healthtech Institutes’s tenth annual drug discovery chemistry, protein–protein interactions conference
Acknowledgments
EG and RZ gratefully acknowledge The Wistar Institute (www.wistar.org) for providing funding in partial support of this work. Also, EG and RZ thank Dr. Elia Eschenazi, Dr. Preston B. Moore and Dr. Vojislava Pophristic at the University of the Sciences, Philadelphia for useful discussions. TEM and PML acknowledge support from the Wellcome Trust Seeding Drug Discovery program (096496/Z/11/Z). TEM acknowledges supports from American Cancer Society (ACS-IRG-96-153-10).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10822_2016_9899_MOESM1_ESM.zip
Supporting Information SI-1: Comparison of apo versus DNA-bound EBNA1 Structures and Figure S1; SI-2 Site Identified by SiteMap and Table S1; SI-3: Molecular Probes used in Binding Sites Identification and Table S2; SI-4 Primary Site or DNA Binding Site and Figure S2; SI-5 Re-docking (3’-TGC-5’)113 against the Primary Site and Figure S3; SI-6 Secondary Site or Recognition Helix Site and Figure S4; SI-7 Simulation Quality Plots: Top AID Hits in Primary Site; SI-8 Simulation Quality Plots: Top AID Hits in Secondary Site; SI-9 Simulation Quality Plots: Fragment substructures in Secondary Site; SI-10 Hydrogen bond networks upon MD simulations of Primary Site hits; SI-11 Hydrogen bond networks upon MD simulations of Secondary Site hits; SI-12 Binding modes and interaction diagrams obtained by induced fit docking of hits against the Secondary Site. IFD scores are listed in Table 2; SI-13 Fragment substructures against the Secondary Site; SI-14 Ligand Efficiency; SI-15 MM-GBSA of Primary Site Hits; SI-16 and SI-17 Attribution of hit 3196499; SI-18 MM-GBSA of Secondary Site Hits. (ZIP 14440 kb)
Rights and permissions
About this article
Cite this article
Gianti, E., Messick, T.E., Lieberman, P.M. et al. Computational analysis of EBNA1 “druggability” suggests novel insights for Epstein-Barr virus inhibitor design. J Comput Aided Mol Des 30, 285–303 (2016). https://doi.org/10.1007/s10822-016-9899-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-016-9899-y