Abstract
Purpose
Feeder cells from animals raise considerable concern for contamination because they are directly in contact with embryonic stem cells.
Methods
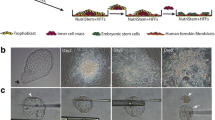
To address this issue we collected discarded foreskin tissue and prepared a fibroblast cell line. We transferred one parthenogenetic blastocyst on to these feeder cells, and later observed outgrowth. By this approach, we were able to derive a human parthenogenetic embryonic stem cell line successfully.
Results
The embryonic stem cells had normal morphology, expressed all expected cell surface markers, could differentiate to embryonic bodies upon culture in vitro, and differentiated further to derivatives of all three germ layers.
Conclusion
This study indicates that homologous human fibroblasts can be used as feeder cells to support not only the propagation, but also the derivation of ES cells, and this should facilitate studies of therapeutic cloning for research and clinical applications.




Similar content being viewed by others
References
Odorico JS, Kaufman DS, Thomson JA. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204.
Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14.
Lin G, OuYang Q, Zhou X, Gu Y, Yuan D, Li W, et al. A highly homozygous and parthenogenetic human embryonic stem cell line derived from a one-pronuclear oocyte following in vitro fertilization procedure. Cell Res. 2007;17:999–1007.
Mai Q, Yu Y, Li T, Wang L, Chen MJ, Huang SZ, et al. Derivation of human embryonic stem cell lines from parthenogenetic blastocysts. Cell Res. 2007;17:1008–19.
Revazova ES, Turovets NA, Kochetkova OD, Kindarova LB, Kuzmichev LN, Janus JD, et al. Patient-specific stem cell lines derived from human parthenogenetic blastocysts. Cloning Stem Cells. 2007;9:432–49.
Kim K, Ng K, Rugg-Gunn PJ, Shieh JH, Kirak O, Jaenisch R, et al. Recombination signatures distinguish embryonic stem cells derived by parthenogenesis and somatic cell nuclear transfer. Cell Stem Cell. 2007;1:346–52.
Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7.
Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6.
Martin GR. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc Natl Acad Sci U S A. 1981;78:7634–8.
Martin MJ, Muotri A, Gage F, Varki A. Human embryonic stem cells express an immunogenic nonhuman sialic acid. Nat Med. 2005;11:228–32.
Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–4.
Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R. Human embryonic stem cells derived without feeder cells. Lancet. 2005;365:1636–41.
Amit M, Margulets V, Segev H, Shariki K, Laevsky I, Coleman R, et al. Human feeder layers for human embryonic stem cells. Biol Reprod. 2003;68:2150–6.
Richards M, Fong CY, Chan WK, Wong PC, Bongso A. Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat Biotechnol. 2002;20:933–6.
Goud PT, Goud AP, Qian C, Laverge H, Van der Elst J, De Sutter P, et al. In-vitro maturation of human germinal vesicle stage oocytes: role of cumulus cells and epidermal growth factor in the culture medium. Hum Reprod. 1998;13:1638–44.
Solter D, Knowles BB. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975;72:5099–102.
Griffith LG, Naughton G. Tissue engineering—current challenges and expanding opportunities. Science. 2002;295:1009–14.
Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki KL, Tzukerman M. Insulin production by human embryonic stem cells. Diabetes. 2001;50:1691–7.
Zhang SC, Wernig M, Duncan ID, Brustle O, Thomson JA. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat Biotechnol. 2001;19:1129–33.
Bradley JA, Bolton EM, Pedersen RA. Stem cell medicine encounters the immune system. Nat Rev Immunol. 2002;2:859–71.
Taylor CJ, Bolton EM, Pocock S, Sharples LD, Pedersen RA, Bradley JA. Banking on human embryonic stem cells: estimating the number of donor cell lines needed for HLA matching. Lancet. 2005;366:2019–25.
Labbe JC, Capony JP, Caput D, Cavadore JC, Derancourt J, Kaghad M, et al. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO J. 1989;8:3053–8.
Drury KC, Schorderet-Slatkine S. Effects of cycloheximide on the “autocatalytic” nature of the maturation promoting factor (MPF) in oocytes of Xenopus laevis. Cell. 1975;4:269–74.
Choi T, Aoki F, Mori M, Yamashita M, Nagahama Y, Kohmoto K. Activation of p34cdc2 protein kinase activity in meiotic and mitotic cell cycles in mouse oocytes and embryos. Development. 1991;113:789–95.
Fulka Jr J, Jung T, Moor RM. The fall of biological maturation promoting factor (MPF) and histone H1 kinase activity during anaphase and telophase in mouse oocytes. Mol Reprod Dev. 1992;32:378–82.
Naito K, Daen FP, Toyoda Y. Comparison of histone H1 kinase activity during meiotic maturation between two types of porcine oocytes matured in different media in vitro. Biol Reprod. 1992;47:43–7.
Naito K, Toyoda Y. Fluctuation of histone H1 kinase activity during meiotic maturation in porcine oocytes. J Reprod Fertil. 1991;93:467–73.
Collas P, Sullivan EJ, Barnes FL. Histone H1 kinase activity in bovine oocytes following calcium stimulation. Mol Reprod Dev. 1993;34:224–31.
Kikuchi K, Naito K, Daen FP, Izaike Y, Toyoda Y. Histone H1 kinase activity during in vitro fertilization of pig follicular oocytes matured in vitro. Theriogenology. 1995;43:523–32.
Kikuchi K, Izaike Y, Noguchi J, Furukawa T, Daen FP, Naito K, et al. Decrease of histone H1 kinase activity in relation to parthenogenetic activation of pig follicular oocytes matured and aged in vitro. J Reprod Fertil. 1995;105:325–30.
Barnes FL, Collas P, Powell R, King WA, Westhusin M, Shepherd D. Influence of recipient oocyte cell cycle stage on DNA synthesis, nuclear envelope breakdown, chromosome constitution, and development in nuclear transplant bovine embryos. Mol Reprod Dev. 1993;36:33–41.
Carroll J. The initiation and regulation of Ca2+ signalling at fertilization in mammals. Semin Cell Dev Biol. 2001;12:37–43.
Li T, Wang S, Xie Y, Lu Y, Zhang X, Wang L, et al. Homologous feeder cells support undifferentiated growth and pluripotency in monkey embryonic stem cells. Stem Cells. 2005;23:1192–9.
Acknowledgements
We are grateful to Michelle Yu for the invaluable collaboration in the writing and revision of the manuscript. This work was supported by grants from the Major State Basic Research Development Program of China (973 Program) (No. 2007CB948001) and the National High Technology Research and Development Program of China (863 Program) (No. 2006AA02A101).
Author information
Authors and Affiliations
Corresponding author
Additional information
Zhenyu Lu, Wanwan Zhu and Yang Yu contributed equally to this work.
Capsule One human embryonic stem cell line from parthenogenetic embryos was derived using human foreskin feeder cells and maintained normal characteristics during long term propagation.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplement Table 1
PCR primers used to detect gene expression in human ES and EB cells (DOC 34 kb)
Supplement Fig. 1
Colony of line P-TJ (passage level 15) growing on the hFF feeder layer. Bar: 100 μm (GIF 218 kb)
Supplement Fig. 2
RT-PCR analysis of the expression of pluripotent genes in line P-TJ (lanes 1–4). The hES cell line H9 was analyzed at the same time as a control (lanes 5–8). Lanes 1, 5: β-actin; lanes 2, 6: OCT4; lanes 3, 7: NANOG; lanes 4, 8: REX1 (GIF 42 kb)
Supplement Fig. 3
Embryoid bodies at day 4 in suspension culture conditions. Bar: 100 μm (GIF 128 kb)
Supplement Fig. 4
T-PCR analysis of the expression of selected imprinted genes. The human parthenogenetic embryonic stem cells expressed only maternal genes (lanes 1, 2), and not paternal genes (lanes 3, 4). The control hES cell line H9 expressed both maternal and paternal genes (lanes 5–8). Lanes 1, 5: H19; lanes 2, 6: UBE3A; lanes 3, 7: hRPNR; lanes 4, 8: IGF2 (GIF 27 kb)
Rights and permissions
About this article
Cite this article
Lu, Z., Zhu, W., Yu, Y. et al. Derivation and long-term culture of human parthenogenetic embryonic stem cells using human foreskin feeders. J Assist Reprod Genet 27, 285–291 (2010). https://doi.org/10.1007/s10815-010-9408-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-010-9408-5