Abstract
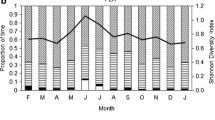
Folivory has evolved in all primate radiations but the relative importance of resource quantity and nutritional quality of food is controversial. To understand how food abundance and different nutrients affect a folivorous diet, we investigated food composition of the leaf-eating lemur Avahi meridionalis. From June to December 2004, we conducted 26 nights of focal observation (256.1 h) in Sainte Luce pluvial littoral forest (southeast Madagascar) and recorded feeding behavior of 4 radiocollared individuals. Within the subjects’ home ranges, we recorded vegetation characteristics (morphospecies, phenology, diameter at breast height) and sampled food and nonfood items for chemical analyses. A. meridionalis did not eat fruit but only leaves and flowers and did not base their choice on food abundance. Adult leaves eaten were higher in easily soluble protein than adult leaves that were not consumed. The subjects ate young leaves and flowers as soon as they became available. These young leaves contained the same concentrations of soluble protein, higher concentrations of crude protein, and lower concentrations of acid detergent fiber and sugar than mature food leaves. A. meridionalis ate leaves with condensed tannins, alkaloids, and intermediate concentrations of polyphenolics. Contrary to previous studies that considered Avahi spp. a specialist, A. meridionalis acted as leaf-eating generalists with moderate selectivity, based on nutritional quality and tolerance of a wide array of plant secondary metabolites. This illustrates the dietary flexibility within a single genus of primates that seems to be driven by environmental constraints rather than morphological or physiological adaptations.




Similar content being viewed by others
References
Altmann, J. (1974). Observational study of behaviour: Sampling methods. Behaviour, 49, 227–267.
Ann, D. K., & Lin, H. H. (1993). Macaque salivary proline-rich saliva: Structure, evolution, and expression. Critical Reviews in Oral Biology and Medicine, 4, 545–551.
Ballhorn, D. J., Kautz, S., & Rakotoarivelo, F. P. (2009). Quantitative variability of cyanogenesis in Cathariostachys madagascariensis—the main food plant of bamboo lemurs in southeastern Madagascar. American Journal of Primatology, 71, 305–315.
Bicca-Marques, J., & Calegaro-Marques, C. (1994). Exotic plant species can serve as a staple food sources for wild howler populations. Folia Primatologica, 63, 209–211.
Bollen, A., & Donati, D. (2005). Phenology of the littoral forest of Sainte Luce, Southeastern Madagascar. Biotropica, 37, 32–43.
Bollen, A., van Elsacker, L., & Ganzhorn, J. U. (2004). Tree dispersal strategies in the littoral forest of Sainte Luce (SE-Madagascar). Oecologia, 139, 604–616.
Campbell, J. L., Eisemann, J. H., Glander, K. E., & Crissey, S. D. (1999). Intake, digestibility, and passage of a commercially designed diet by two Propithecus species. American Journal of Primatology, 48, 237–246.
Campbell, J. L., Eisemann, J. H., Williams, C. V., & Glenn, K. M. (2000). Description of the gastrointestinal tract of five lemur species: Propithecus tattersalli, Propithecus verreauxi coquereli, Varecia variegata, Hapalemur griseus, and Lemur catta. American Journal of Primatology, 52, 133–142.
Campbell, J. L., Williams, C. V., & Eisemann, J. H. (2004). Use of total dietary fiber across four lemur species (Propithecus verreauxi coquereli, Hapalemur griseus, Varecia variegata, and Eulemur fulvus): Does fiber type affect digestive efficiency? American Journal of Primatology, 64, 323–335.
Carrai, V., Borgognini-Tarli, S. M., Huffman, M. A., & Bardi, M. (2003). Increase in tannin consumption by sifaka (Propithecus verreauxi verreauxi) females during the birth season: A case for self-medication in prosimians? Primates, 44, 61–66.
Chapman, C. A., Chapman, L. J., Bjorndal, K. A., & Onderdonk, D. A. (2002). Application of protein-to-fiber ratios to predict colobine abundance on different spatial scales. International Journal of Primatology, 23, 283–310.
Chapman, C. A., Chapman, L. J., Naughton-Treves, L., Lawes, M. J., & Mc Dowell, L. R. (2004). Predicting folivorous primate abundance: Validation of a nutritional model. American Journal of Primatology, 62, 55–69.
Chivers, D. J., & Hladik, C. M. (1980). Morphology of the gastrointestinal tract in primates: Comparison with other mammals in relation to diet. Journal of Morphology, 166, 337–386.
Cork, S. J. (1996). Optimal digestive strategies for arboreal herbivorous mammals in contrasting forest types. Why koalas and colobines are different. Australian Journal of Ecology, 21, 10–20.
Cromwell, B. T. (1955). The alkaloids. In K. Paech & M. V. Tracy (Eds.), Modern methods of plant analysis (pp. 367–374). Heidelberg: Springer.
Dasilva, G. L. (1994). The western black-and-white colobus as a low-energy strategist: Activity budgets, energy expenditure and energy intake. Journal of Animal Ecology, 61, 79–91.
Davies, A. G., Bennett, E. L., & Waterman, P. G. (1988). Food selection by two Southeast Asian colobine monkeys (Presbytis rubicunda and Presbytis melalophos) in relation to plant chemistry. Biological Journal of the Linnean Society, 34, 33–56.
Dearing, M. D., Mangione, A. M., & Karasov, W. H. (2000). Diet breadth of mammalian herbivores: Nutrient vs. detoxification constraints. Oecologia, 123, 397–405.
DeGabriel, J. L., Wallis, I. R., Moore, B. D., & Foley, W. J. (2008). A simple, integrative assay to quantify nutritional quality of browses for herbivores. Oecologia, 156, 107–116.
Demment, M. W., & van Soest, P. J. (1985). A nutritional explanation for body-size patterns of ruminant and nonruminant herbivores. American Naturalist, 125, 641–672.
Dewar, R. E., & Richard, A. F. (2007). Evolution in the hypervariable environment of Madagascar. Proceedings of the National Academy of Sciences of the USA, 104, 13723–13727.
Dial, K. P. (1988). Three sympatric species of Neotoma—Dietary specialization and coexistence. Oecologia, 76, 531–537.
Donati, G., & Borgognini-Tarli, S. M. (2006). The influence of abiotic factors on cathemerality: The case of Eulemur fulvus collaris in the littoral forest of Madagascar. Folia Primatologica, 77, 104–122.
Duncan, A. J., & Gordon, I. J. (1999). Habitat selection according to the ability of animals to eat, digest and detoxify foods. Proceedings of the Nutrition Society, 58, 799–805.
Edwards, M. S., & Ulrey, D. E. (1999). Effect of dietary fiber concentration on apparent digestibility and digesta passage in non-human primates. II. Hindgut- and foregut-fermenting folivores. Zoo Biology, 18, 537–549.
Eppley, T. M., Verjans, E., & Donati, G. (2011). Coping with low-quality diets: A first account of the feeding ecology of the southern gentle lemur, Hapalemur meridionalis, in the Mandena littoral forest, southeast Madagascar. Primates, 52, 7–13.
Faulkner, A. L., & Lehman, S. M. (2006). Feeding patterns in a small-bodied nocturnal folivore (Avahi laniger) and the influence of leaf chemistry: A preliminary study. Folia Primatologica, 77, 218–227.
Felton, A. M., Felton, A., Lindenmayer, D. B., & Foley, W. J. (2009). Nutritional goals of wild primates. Functional Ecology, 23, 70–78.
Fleagle, J. G. (1998). Primate adaptation and evolution. Orlando, FL: Academic Press.
Foley, W. J., & Cork, S. J. (1992). Use of fibrous diets by small herbivores: How far can the rules be ‘bent’? Trends in Ecology & Evolution, 7, 159–162.
Foley, W. J., Iason, G., & McArthur, C. (1999). Role of plant secondary metabolites in the nutritional ecology of mammalian herbivores—how far have we come in 25 years? In H. J. G. Jung & G. C. Fahey Jr. (Eds.), Vth International Symposium on the Nutrition of Herbivores (pp. 203–274). Savoy, IL: American Society of Animal Science.
Freeland, W. J. (1991). Plant secondary metabolites: Biochemical coevolution with herbivores. In R. T. Palo, R. T. Palo, & C. T. Robbins (Eds.), Plant defenses against mammalian herbivory (pp. 61–81). Boca Raton, FL: CRC Press.
Freeland, W. J., & Janzen, D. H. (1974). Strategies in herbivory by mammals: The role of plant secondary compounds. American Naturalist, 108, 269–289.
Ganzhorn, J. U. (1988). Food partitioning among Malagasy primates. Oecologia, 75, 436–450.
Ganzhorn, J. U. (1989). Niche seperation of seven lemur species in the eastern rainforest of Madagascar. Oecologia, 79, 279–286.
Ganzhorn, J. U. (1992). Leaf chemistry and the biomass of folivorous primates in tropical forests. Test of a hypothesis. Oecologia, 91, 540–547.
Ganzhorn, J. U. (2002). Distribution of a folivorous lemur in relation to seasonally varying food resources: Integrating quantitative and qualitative aspects of food characteristics. Oecologia, 131, 427–435.
Ganzhorn, J. U., & Abraham, J.-P. (1991). Possible role of plantations for lemur conservation in Madagascar: Food for folivorous species. Folia Primatologica, 56, 171–176.
Ganzhorn, J. U., & Wright, P. C. (1994). Temporal patterns in primate leaf eating: The possible role of leaf chemistry. Folia Primatologica, 63, 203–208.
Ganzhorn, J. U., Abraham, J. P., & Razanahoera-Rakotomalala, M. (1985). Some aspects of the natural history and food selection of Avahi laniger. Primates, 26, 452–463.
Glander, K. E. (1982). The impact of plant secondary compounds on primate feeding behavior. Yearbook of Physical Anthropology, 25, 1–18.
Glander, K. E., Wright, P. C., Seigler, D. S., Randrianasolo, V., & Randrianasolo, B. (1989). Consumption of cyanogenic bamboo by a newly discovered species of bamboo lemur. American Journal of Primatology, 19, 119–124.
Gouvenain, R. C., & Silander, J. A., Jr. (2003). Littoral forest. In S. M. Goodman & J. P. Benstead (Eds.), The natural history of Madagascar (pp. 103–111). Chicago: University of Chicago Press.
Grassi, C. (2006). Variability in habitat, diet, and social structure of Hapalemur griseus in Ranomafana National Park, Madagascar. American Journal of Physical Anthropology, 131, 50–63.
Harcourt, C. (1991). Diet and behaviour of a nocturnal lemur, Avahi laniger, in the wild. Journal of Zoology London, 223, 667–674.
Hemingway, C. A. (1998). Selectivity and variability in the diet of Milne-Edwards’ sifakas (Propithecus diadema edwardsi): Implications for folivory and seed-eating. International Journal of Primatology, 19, 355–377.
Hemingway, C. A., & Bynum, N. (2005). The influence of seasonality on primate diet and ranging. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates: Studies of living and extinct human and non-human primates (pp. 57–104). Cambridge, UK: Cambridge University Press.
Hoeck, H. N. (1989). Demography and competition in hyrax. A 17 years study. Oecologia, 79, 353–360.
Huffman, M. A., & Chapman, C. A. (2009). Primates and their parasites. Cambridge, UK: Cambridge University Press.
Iason, G. (2005). The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proceedings of the Nutrition Society, 64, 123–131.
Irwin, M. T. (2006). Ecologically enigmatic lemurs: The sifakas of the eastern forests (Propithecus candidus, P. diadema, P. edwardsi, P. perrieri, and P. tattersalli). In L. Gould & M. Sauther (Eds.), Lemurs: Ecology and adaptation (pp. 305–326). New York: Springer.
Janson, C. H., & Chapman, C. (1999). Resources and primate community structure. In J. G. Fleagle, C. H. Janson, & K. Reed (Eds.), Primate communities (pp. 237–267). Cambridge, UK: Cambridge University Press.
Johns, A. (1986). Effects of selective logging on the behavioural ecology of west Malaysian primates. Ecology, 67, 684–694.
Kay, R. F. (1984). On the use of anatomical features to infer foraging behavior in extinct primates. In P. S. Rodman & J. G. H. Cant (Eds.), Adaptations for foraging in nonhuman primates: Contributions to an organismal biology of prosimians, monkeys and apes (pp. 21–53). New York: Columbia University Press.
Keeley, J. E., & Fotheringham, C. J. (2005). Plot shape effects on plant species diversity measurements. Journal of Vegetation Science, 16, 249–256.
Krief, S., Huffman, M. A., Sevenet, T., Hladik, C. M., Grellier, P., Loiseau, P. M., & Wrangham, R. W. (2006). Bioactive properties of plant species ingested by chimpanzees (Pan troglodytes schweinfurthii) in the Kibale National Park, Uganda. American Journal of Primatology, 68, 51–71.
Lehman, S. (2007). Ecological and phylogenetic correlates to body size in the Indriidae. International Journal of Primatology, 28, 183–210.
Mackie, R. I. (2002). Mutualistic fermentative digestion in the gastrointestinal tract: Diversity and evolution. Integrative and Comparative Biology, 42, 319–326.
Marsh, K. J., Wallis, I. R., Andrew, R. L., & Foley, W. J. (2006). The detoxification limitation hypothesis: Where did it come from and where is it going? Journal of Chemical Ecology, 32, 1247–1266.
Martin, R. D. (1990). Primate origins and evolution. Princeton, NJ: Princeton University Press.
Mattson, W. J. (1980). Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics, 11, 119–161.
McArthur, C., Sanson, G. D., & Beal, A. M. (1995). Salivary proline-rich proteins in mammals: Roles in oral homeostasis and counteracting dietary tannins. Journal of Chemical Ecology, 21, 663–691.
McKey, D. B., Waterman, P. G., Gartlan, J. S., & Struhsaker, T. T. (1978). Phenolic content of vegetation in two African rain forests: Ecological implications. Science, 202, 61–64.
Mehansho, H., Butler, L. G., & Carlson, D. M. (1987). Dietary tannins and salivary proline-rich proteins: Interactions, induction and defense mechanisms. Annual Review of Nutrition, 7, 423–440.
Milton, K. (1979). Factors influencing leaf choice by howler monkeys: A test of some hypotheses of food selection by generalist herbivores. American Naturalist, 114, 362–378.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): Energetic and digestive considerations and comparison with the Colobinae. International Journal of Primatology, 19, 513–547.
Moore, B. D., & Foley, W. J. (2000). A review of feeding and diet selection in koalas (Phascolarctos cinereus). Austalian Journal of Zoology, 48, 317–333.
Moore, B. D., & Foley, W. J. (2005). Tree use by koalas in a chemically complex landscape. Nature, 435, 488–490.
Mutschler, T. (1999). Folivory in a small-bodied lemur. The nutrition of the Aloatra Gentle lemur (Hapalemur griseus alaotrensis). In B. Rakotosamimanana, H. Rasamimanana, J. U. Ganzhorn, & S. M. Goodman (Eds.), New directions in lemur studies (pp. 221–239). New York: Kluwer Academic/Plenum Press.
Nash, L. T. (1998). Vertical clingers and sleepers: Seasonal influences on the activities and substrate use of Lepilemur leucopus at Beza Mahafaly special reserve, Madagascar. Folia Primatologica, 69, 204–217.
National Research Council (NRC). (2003). Nutrient requirements of non-human primates, 2nd rev. ed. Washington, DC: National Academies Press.
Norscia, I. (2008). Pilot survey of avahi population (woolly lemurs) in littoral forest fragments of southeast Madagascar. Primates, 49, 85–88.
Norscia, I., & Borgognini-Tarli, S. M. (2008). Ranging behavior and possible correlates of pair-living in southeastern avahis (Madagascar). International Journal of Primatology, 29, 153–171.
Norscia, I., Carrai, V., & Borgognini-Tarli, S. M. (2006). Influence of dry season and food quality and quantity on behavior and feeding strategy of Propithecus verreauxi in Kirindy, Madagascar. International Journal of Primatology, 27, 1001–1022.
Oates, J. F., Whitesides, G. H., Davies, A. G., Waterman, P. G., Green, S. M., Dasilva, G. L., & Mole, S. (1990). Determinants of variation in tropical forest primate biomass: New evidence from West Africa. Ecology, 71, 328–343.
Oksanen, L. (1992). Evolution of exploitation ecosystems. I. Predation, foraging ecology and population dynamics in herbivores. Evolutionary Ecology, 6, 15–23.
Pavelka, M. S. M., & Knopff, K. H. (2004). Diet and activity in black howler monkeys (Alouatta pigra) in southern Belize: Does degree of frugivory influence activity level? Primates, 45, 105–111.
Powzyk, J. A., & Mowry, C. B. (2003). Dietary and feeding differences between sympatric Propithecus diadema diadema and Indri indri. International Journal of Primatology, 24, 1143–1162.
Shimada, T. (2006). Salivary proteins as a defense against dietary tannins. Journal of Chemical Ecology, 32, 1149–1163.
Shipley, L. A., Forbey, J. S., & Moore, B. D. (2009). Revisiting the dietary niche: When is a mammalian herbivore a specialist? Integrative and Comparative Biology, 49, 274–290.
Siegel, S., & Castellan, N. J., Jr. (1988). Nonparametric statistics for the behavioral sciences (2nd ed.). New York: McGraw-Hill.
Silver, S. C., Ostro, L. E. T., Yeager, C. P., & Horwich, R. (1998). Feeding ecology of black howler monkey (Alouatta pigra) in Northern Belize. American Journal of Primatology, 45, 263–279.
Skopec, M. M., Haley, S., Torregossa, A. M., & Dearing, M. D. (2008). An oak (Quercus agrifolia) specialist (Neotoma macrotis) and a sympatric generalist (Neotoma lepida) show similar intakes and digestibility of oak. Physiological and Biochemical Zoology, 81, 426–433.
Stolter, C., Niemelä, P., Varvikko, T., Julkunen-Tiitto, R., Ball, J. P., Vanhatalo, A., Danell, K., & Ganzhorn, J. U. (2009). Comparison of secondary chemistry and digestibility of three different boreal coniferous trees. Basic and Applied Ecology, 10, 19–26.
Tan, C. L. (1999). Group composition, home range size, and diet of three sympatric bamboo lemur species (genus Hapalemur) in Ranomafana National Park, Madagascar. International Journal of Primatology, 20, 547–566.
Thalmann, U. (2001). Food resources in two nocturnal lemurs with different social behavior: Avahi occidentalis and Lepilemur edwardsi. International Journal of Primatology, 22, 287–324.
Torregrossa, A.-M., Azzara, A. V., & Dearing, M. D. (2011). Testing the diet breadth trade-off hypothesis: Differential regulation of novel plant secondary compounds by a specialist and a generalist herbivore. Oecologia.
van Soest, P. J. (1996). Allometry and ecology of feeding behavior and digestive capacity in herbivores: A review. Zoo Biology, 15, 455–479.
Villalba, J. J., Provenca, F. D., & Shaw, R. (2006). Sheep self-medicate when challenged with illness-inducing foods. Animal Behaviour, 71, 1131–1139.
Wallis, I. R., Edwards, M. J., Windley, H., Krockenberger, A. K., Felton, A., Quenzer, M., Ganzhorn, J. U., & Foley, W. J. (2011). Food for folivores—nutritional explanations linking diets to population density. Oecologia.
Warren, R. D. (1997). Habitat use and support preference of two free-ranging saltatory lemurs (Lepilemur edwardsi and Avahi occidentalis). Journal of Zoology (London), 241, 325–341.
Warren, R. D., & Crompton, R. H. (1997). Locomotor ecology of Lepilemur edwardsi and Avahi occidentalis. American Journal of Physical Anthropology, 104, 471–486.
Warren, R. D., & Crompton, R. H. (1998). Diet, body size and the energy costs of locomotion in saltatory primates. Folia Primatologica, 69, 86–100.
Westoby, M. (1974). An analysis of diet selection by large generalist herbivores. American Naturalist, 108, 290–304.
Westoby, M. (1978). What are the biological bases of varied diets? American Naturalist, 112, 627–631.
Wiggins, N. L., McArthur, C., & Davies, N. W. (2006). Diet switching in a generalist mammalian folivore: Fundamental to maximising intake. Oecologia, 147, 650–657.
Willmer, P., Stone, G., & Johnston, I. A. (2000). Environmental physiology of animals. Malden, MA: Wiley-Blackwell.
Wright, P. C. (1999). Lemur traits and Madagascar ecology: Coping with an island environment. Yearbook of Physical Anthropology, 42, 31–72.
Yamashita, N. (2008). Chemical properties of the diets of two lemur species in southwestern Madagascar. International Journal of Primatology, 29, 339–364.
Yamashita, N., Tan, C. L., Vinyard, C. J., & Williams, C. (2010). Semi-quantitative tests of cyanide in foods and excreta of three Hapalemur species in Madagascar. American Journal of Primatology, 72, 56–61.
Yeager, C. P., & Kirkpatrick, R. C. (1998). Asian colobine social structure: Ecological and evolutionary constraints. Primates, 39, 147–155.
Zaramody, A., Fausser, J. L., Roos, C., Zinner, D., Andriaholinirina, N., Rabarivola, C., Norscia, I., Tattersall, I., & Rumpler, Y. (2006). Molecular phylogeny and taxonomic revision of the eastern woolly lemurs (Avahi laniger). Primate Report, 74, 9–22.
Acknowledgments
We thank the Commission Tripartite du Département des Eaux et Forêt, the Parque Botanique et Zoologique de Tsimbasasa, the Université d’Antananarivo, and Silvana M. Borgognini for research permits and support; Qit Madagascar Minerals (QMM) for providing logistic support; the QMM conservation staff: Manon Vincelette for research follow-up and Faly Kandriantafika and David Rabehevitra for botanical identification of plant species; Givet, Kadoff, Roline, and Josette for their excellent help in the field; the people of the villages of Ambandriky and S. Luce for helping in case of need. Irene Tomaschewski did a wonderful job for plant analyses. We also thank Elisabetta Palagi and the anonymous reviewers for their thorough and thoughtful suggestions. Joanna Setchell did an outstanding job providing excellent guidance and editing the manuscript. This research is part of the Ph.D. thesis of I. Norscia partly funded by MIUR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Norscia, I., Ramanamanjato, J.B. & Ganzhorn, J.U. Feeding Patterns and Dietary Profile of Nocturnal Southern Woolly Lemurs (Avahi meridionalis) in Southeast Madagascar. Int J Primatol 33, 150–167 (2012). https://doi.org/10.1007/s10764-011-9562-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-011-9562-3