Abstract
Benzene, a recognized occupational leukemogen in adults, has been hypothesized to also increase the risk of childhood leukemia. We carried out a population-based case–control study in a northern Italy community involving 83 cases with acute childhood leukemia diagnosed in the years 1998–2009 and 332 matched controls. We assessed residential exposure to benzene and to particulate matter ≤10 μm (PM10) from motorized traffic using geocoded residences and detailed emission and dispersion modeling. Exposure to benzene, and to a lesser extent to PM10, appeared to be independently associated with an excess leukemia risk. When we stratified the study population by age and by leukemia subtype, the relative risk associated with benzene exposure was higher among children aged less than 5 years, and despite small numbers this relation appeared to be considerably stronger for acute myeloid leukemia than for acute lymphoblastic leukemia. Overall, these findings suggest that exposure to low levels of benzene released from motorized traffic may increase the risk of childhood leukemia, and suggest a possible independent effect of PM10, although unmeasured confounding due to other pollutants cannot be ruled out.
Similar content being viewed by others
Background
Benzene has been recognized as a leukemogen, for acute myeloid leukemia in adults and has been associated with other disease subtypes including chronic lymphocytic leukemia and childhood leukemia [1–6]. Increased leukemia risk has been reported at very low environmental levels of benzene [7, 8]. Emissions from motorized traffic, which include benzene, have been hypothesized to increase the risk of childhood leukemia, based on results from recent epidemiologic and toxicological studies [3, 9–13]. This relation, however, is far from accepted, because (1) there are few studies; (2) some studies show null results [14]; (3) there may be uncontrolled confounding; (4) results are generally imprecise; (5) in many locations environmental benzene concentrations have decreased while disease rates have increased; and (6) exposure assessments have had significant limitations. Numerous factors influence ambient air concentrations of pollutants emitted from vehicular traffic. These include diurnal variation in the number and types of vehicles on roads, fuel types, local meteorology, seasonal variation in meteorological conditions, and contributions of emissions from multiple roadways [15–17]. Another important factor is the rapid decrease in concentrations of pollutants emitted by motorized traffic with distance from the roadway, which makes accurate location information important. Few studies have been able to address all these issues, and none seems to have considered the independent effect of benzene taking into account another pollutant of major importance, respirable particulate matter, a key source of exposure to adsorbed toxic and carcinogenic contaminants such as polycyclic aromatic hydrocarbons and heavy metals [18–21].
To address these issues, we conducted a case–control study of childhood leukemia in a northern Italy population that included detailed exposure assessment based on modeling ambient air concentrations of benzene from traffic emissions at the geocoded residence of each subject, together with assessment of exposure to PM10 (particles with a diameter of 10 μm or less).
Methods
Subjects
This study was a population-based case–control study carried out in the provinces of Modena and Reggio Emilia, both located in the Emilia-Romagna region of northern Italy. These neighboring provinces have a population of about 1,200,000 inhabitants who are homogeneous with respect to ethnicity and socioeconomic status. The cases comprised all children aged 0–14 diagnosed with leukemia while residing in Modena or Reggio Emilia during the years 1998–2009. Cases were identified using the hospital-based registry of the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) [22], to which children diagnosed with neoplasms admitted to nearly all Italian hospitals are referred. In the Emilia-Romagna region all four hospitals that treat pediatric neoplasms are included in the AIEOP Registry. Fourteen patients residing in 32 sparsely populated mountain municipalities were excluded from the analysis because of difficulties related to exposure assessment (see following section). The registry database allowed identification of leukemia subtype. Residence at diagnosis was retrieved from administrative databases, mainly consisting of hospital discharge or clinical records.
The control series comprised four subjects matched to each case for sex, year of birth and province of residence during the diagnosis year. Controls were obtained from population data from the National Health Services Local Health Units of Modena and Reggio Emilia, which record all residents annually, by randomly sampling four children among possible matches. For Modena province, no historical population database was available for years before 2005. Therefore, for the selection of Modena province controls for years 1998–2004, we used the 2005 database, and verified actual residence in the year of diagnosis of the matched case through the Revenue Agency of the Ministry of Finance, which maintains records of historical residence nationwide. Additional controls were selected when one or more of the initial selections resided outside the province. Subjects residing in the mountain municipalities located in the southern part of the two provinces were excluded from the population databases before carrying out the sampling procedure. Access to the Ministry of Finance database also allowed us to retrieve annual gross income of parents of the study subjects, which was used to compute family income category for the index year.
Exposure assessment
We assessed benzene and PM10 exposure for each study subject by geocoding his/her residential address and modeling ambient air concentrations at this location. We geocoded residences of cases at date of diagnosis and of controls in the corresponding year using ARC-GIS software (version 9.2, ESRI, Redlands, CA 2006). To geocode addresses we used a database of satellite coordinates made available by Modena and Reggio Emilia provinces or, for addresses not included in the database, Google Earth or a direct in loco measure using a portable GPS device (GPSmap 60CSx, Garmin Int. Corp., Olathe, KS). Geocoding also allowed, for subjects residing in the two main study area cities (Modena and Reggio Emilia), evaluation of exposure to magnetic fields at intensity ≥0.1 μT generated from high-voltage power lines, using previously described methodology [23].
The CAlifornia LINE Source Dispersion Model, version 4 (CALINE4), a line source air quality model developed by the California Department of Transportation, was used to model the dispersion of emissions from vehicular traffic. CALINE4 is a stationary plume dispersion model for roads and other linear sources that is used to estimate the dispersion and deposition of pollutants such as carbon monoxide, particulate matter, nitrogen dioxide, benzene and other contaminants at predefined spatial receptors [24]. We entered benzene emissions from vehicular traffic estimated from traffic flows on the main roads of the province. The model was applied over a full year, to encompass daily, weekly, and seasonal variation in weather and traffic conditions. The model predicted hourly benzene concentrations at the location of residence of each study subject at a height of 2 meters. We summarized the final output of the model as an average concentration and a maximum hourly concentration.
We used estimates of traffic flow from previously conducted studies for the province of Modena for the year 2006 and for the province of Reggio Emilia for 2005 [25, 26]. The traffic flow estimates were generated using a model that incorporated demographic and occupational information for all residents of the provinces, and detailed personal mobility information collected by the National Institute of Statistics 2001 Census and validated through surveys and with automatic vehicles counters. The model created a matrix of vehicle movements for each road, on the basis of daily movements estimated for their residents taking into account their age, sex, family structure and occupation [25, 26]. For Reggio Emilia, these data were further validated by a survey of randomly selected families and car drivers carried out in 2005 by the Department of Planning of Venice University, coordinated by one of the authors (A.M.) [26].
We computed emissions using emission factors for light and heavy vehicles and for urban and suburban areas. The emission factors for benzene were derived from a 1990–2007 transport database developed by the Italian National Institute for Environmental Protection and Research (www.isprambiente.gov.it) and calculated using the program COPERT IV developed by the Laboratory of Applied Thermodynamics of the Aristotle University of Thessaloniki (www.emisia.com/copert/General.html). The COPERT IV emission factors are detailed by directive reference, engine capacity, weight class and fuel, for different vehicles classes (passenger cars, light duty vehicles, heavy duty vehicles, urban buses and coaches, two wheelers) and tabulated according to the driving cycle (urban, suburban and highway, given the dependence of the emission factors of vehicle speed). Mean values of benzene emission factors (calculated from the number of vehicles registered and from the relative annual average mileage) were 23.5 and 0.82 mg/km for light and heavy vehicles, respectively, in the urban cycle, and 2.96 and 0.31 mg/km for rural cycle.
Meteorological data were obtained using a meteorological model, CALMET, deployed at Hydro Meteorological Service of the Emilia-Romagna environmental protection agency ARPA—Agenzia Regionale per la Protezione Ambientale (www.arpa.emr.it/cms3/documenti/_cerca_doc/meteo/ambiente/descrizione_calmet.pdf). This model elaborates, on the basis of measured data, parameters such as temperature, wind speed and direction, stability class and height of the mixing layer.
Using the above data, we used CALINE4 to estimate average benzene concentration for each hour of the simulation year at each study subject’s location. As suggested by the CALINE4 Technical Guide [24], we ran the model increasing the road width by 3 meters to the right and left, in order to account for thermal and mechanical turbulence caused by vehicles. We removed from further analysis the mountain areas located in the southern part of the two provinces, due to rough topography, which cannot be taken in account by CALINE4. Less than 10 % of the total population of the two provinces resided in the excluded area.
Some simplifications were incorporated into the modeling. We did not consider the effect of additional turbulence created by tall buildings (urban canyons) because we lacked information about building height. Since the calculation domain was located mainly in the flat area of the two provinces, the land was considered to be flat. No additional emission sources such as industrial solvent use, parking lots or petrol stations were considered.
We modeled ambient air concentrations of PM10 released from traffic using the same methodology as described for benzene. PM10 emission factors include both exhaust (emissions from tailpipe, obtained from ISPRA database based on COPERT IV calculations) and non-exhaust components (abrasion and resuspension processes, obtained from Gehrig determination [27]). Values of PM10 emissions factors used in our calculations were 105.7 and 1,054.6 mg/km for light and heavy vehicles, respectively, in the urban cycle, and 62.3 and 337.0 mg/km for rural cycle.
To validate the results, we ran the CALINE4 model with reference to 3 air quality monitoring stations in Reggio Emilia in 2005 and 4 monitoring stations in Modena in 2006; one station was excluded due to unreliability of traffic data for that location. The Pearson correlation coefficient between the estimated (modeled) and measured yearly mean levels (maximum measured levels were not available for technical reasons) was 0.43 (95 % CI −0.48–0.89) for benzene and for 0.64 (95 % CI −0.21–0.94) for PM10. Historical data from these monitoring stations were also used to characterize trends in ambient air levels of benzene over the study period; results indicated a decrease in benzene concentrations over time, with average concentrations in the 1998–2003 period about twice the values for 2004–2009; for the only Modena city monitoring station operating throughout the entire study period, for example, values averaged 5.2 μg/m3 for 1998–2003 and 2.2 μg/m3 for 2004–2009. This decrease is consistent with reduction in the benzene content of gasoline over this time period as well as a change in the composition of the vehicle fleet towards lower emission vehicles.
Statistical analysis
Analyses were conducted using bivariate and multivariate conditional logistic regression models and generalized additive models [28]. To examine the sensitivity of the findings to model specification, we entered exposure in the models using both categorical cutpoints and continuous variables, with the latter modeled as both untransformed and log-transformed. Each generalized additive model consisted of a logistic regression model in which the relation between the log odds of being a case and the exposure variable was modeled nonparametrically using a natural cubic spline, with control for the matching variables (sex, age, year of diagnosis and province) and the other pollutant. Since the distributions of the exposure variables (mean benzene and mean PM10 concentrations) were right-skewed with outliers at high exposure levels, log transformations were used to achieve approximately symmetric distributions and reduce the influence of outliers. To improve interpretability, we used a log base 10 transformation, so that a one-unit increase in the log-transformed variable is equivalent to a tenfold increase in exposure (e.g., 0.05 vs. 0.5 μg/m3). These analyses were conducted using the gam package in R version 2.9.2 [29]. Other analyses were conducted using Stata 12.1 (Stata Corp. College Station, TX, 2012). We conducted analysis stratified by age of diagnosis (<5 vs. ≥5 years) to explore possible age-related susceptibilities and to minimize the effect of exposure misclassification due to antecedent changes of residence, and subgroups analyses for acute lymphoblastic leukemia and acute myeloid leukemia.
Results
During the study period, 83 cases of acute childhood leukemia (50 males and 33 females) occurred in the study area, including 64 cases of lymphoblastic leukemia (average age at diagnosis 5.1, standard deviation 4.8 years) and 19 of other leukemia types included in the myeloid category (6.6 ± 3.8 years). The distribution of estimated annual average and maximum hourly benzene and PM10 concentrations in outdoor ambient air are reported in Table 1. For the majority of subjects, the estimated average benzene and PM10 exposure from vehicular traffic were considerably lower than the European Union (EU) standards for ambient air of 5 and 40 μg/m3, respectively. Mean concentrations of benzene and PM10 were moderately associated (Pearson correlation coefficient 0.53).
In analyses with exposure categories based on cutpoints yielding nearly equal sized number of controls in four categories, relative risk (RR) values, as estimated through the odds ratios, were elevated in the highest category of benzene exposure, for both average and maximum hourly concentration, both in the crude analysis and when adjusted for average PM10 exposure (Table 2). Further adjustment for parental income and, in the urban area of Modena and Reggio Emilia, for exposure to magnetic fields from high-voltage power lines, or assigning exposure status according to exact quartile cutpoints of pollutants exposure among controls, had little effect on the relative risk estimates (data not shown). RR values were also elevated in the two highest categories of average PM10 levels, and remained elevated after adjustment for average benzene concentration (Table 2). There was no indication of increased risk associated with maximum hourly PM10 levels.
Results of analyses stratified by age of diagnosis of case (<5 years and ≥5 years) using approximate quartiles of exposure are presented in Table 3. In the younger group, there was an increased RR associated with the third and particularly the fourth quartile of mean benzene exposure, adjusting for PM10. RR estimates for PM10 adjusted for benzene exposure were also consistent with a dose–response relation. For children aged 5 years or older, RRs associated with average benzene exposure were near unity in all exposure categories, while RRs were higher for average PM10 in the third and fourth quartiles of exposure.
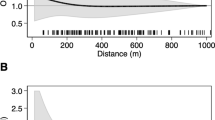
Nonparametric natural cubic spline regression analysis (Fig. 1), with adjustment for the matching variables, suggested a positive association between average benzene exposure and leukemia risk in the overall population, with adjustment for PM10. In age-stratified analyses, there was a strong positive association among children younger than 5 years, and suggestion of a negative association among children 5 years or older. Results for PM10 also suggested a possible positive association with leukemia risk in the population overall. In age-stratified analyses, there was little evidence of an association among children younger than 5 years but a possible positive association among children 5 years and older (Fig. 1).
Natural cubic spline models from generalized additive model for the relation between log odds of case status and mean benzene and between case status and PM10 exposure, controlling for sex, age, year of diagnosis, province and the other pollutant. Rugplots at the bottom of each plot provide the distribution of exposure levels of subjects
Since the nonparametric analyses suggested that the log odds might be linear in the log-transform of each exposure variable, RRs were estimated for a conventional parametric conditional logistic regression model using log-transformed exposure variables (Table 4), in addition to untransformed values to assess sensitivity of the findings to model specification. In the overall population, a one-unit log base 10-transformed increase in benzene exposure, corresponding to a tenfold increase in mean benzene concentration, was associated with a RR of 1.37 (95 % CI 0.83–2.25), while the corresponding RR for PM10 was 1.51 (95 % CI 0.71–3.20). Adjustment for income increased the RRs slightly, while adjustment for the other pollutant attenuated the RR estimates somewhat. Estimates computed using the exposure variables on their original untransformed scale yielded roughly comparable results.
When stratified by age, a much higher RR of disease associated with benzene exposure was found for children younger than 5 years, with a RR of 2.72 (95 % CI 1.23–6.01), compared with older children, who had a RR of 0.86 (95 % CI 0.49–1.52). Higher RRs among younger subjects also emerged for PM10 but were attenuated after adjustment for benzene exposure. Analyses carried out using the crude benzene and PM10 values yielded substantially similar results.
Subgroup analysis according to leukemia type showed much higher estimates, though statistically very unstable, for myeloid leukemia than for lymphoblastic leukemia in the youngest age group (Table 5).
Discussion
Our results suggest the benzene exposure from vehicular traffic at levels lower than the current European Union limit of 5 μg/m3 [30] may have been associated with increased risk of childhood leukemia in this Italian population, particularly for children under 5 years of age. Confounding due to socioeconomic factors, magnetic fields exposure (as suggested by Langholtz et al. [14]) and PM10 from vehicular traffic did not appear to explain these findings, though the only variable available to assess parental socioeconomic status might not have controlled it adequately. PM10 was also associated with elevated RRs in children under the age of 5, but this association was attenuated when adjustment was made for benzene exposure. These results for benzene are consistent with some previously reported epidemiologic studies that examined the relation between exposure from motorized traffic and childhood leukemia risk [12, 31] and with evidence concerning adult lymphoid leukemia and other lymphoid malignancies [3, 7]. Maximum pollutant exposure did not yield additional evidence of a relation with the excess leukemia risk compared with average levels, and this was particularly true for PM10, suggesting that usual exposure, the one generally monitored by regulatory agencies, is not less informative than peak exposures in influencing disease risk.
This study has important limitations. The study design did not allow for collection of information directly from subjects and their families, so there is limited information about potential confounders. On the other hand, available evidence from the literature has not consistently identified major risk factors for childhood leukemia, apart from ionizing and non-ionizing radiation and possibly genetic susceptibility [31–33], and there is no reason to hypothesize a different prevalence of these variables across benzene exposure subgroups in our study. Moreover, contacting study subjects would have created a risk of selection and recall bias, and the inclusion of family income and matching on age, sex, year of diagnosis and province should have reduced the risk of confounding due to unmeasured factors. We used modeled ambient air levels to estimate study subjects’ exposure. There were multiple possible sources of error in the exposure assessment, including the emission factors utilized, the vehicle traffic estimates and the use of a single calendar year to represent exposures that occurred at various times during the study period. Owing to the limited number of air monitoring stations available, we had limited ability to validate the modeling, and our validation suggested only moderate correlation between modeled and measured ambient air levels. Errors in measurement of exposure could have introduced bias and imprecision into the effect estimates. However, the measurement error was likely to have been non-differential with respect to disease status and thus could be expected to bias risk estimates towards the null. We attempted to isolate the effect of single agents (benzene and PM10) whereas vehicular exhaust is a complex mixture of agents, and unmeasured confounding due to other pollutants may have occurred. We used the residential location at time of diagnosis (or in the corresponding year for referents) to assess benzene exposure for study subjects, an approach that might not have adequately accounted for antecedent exposure due to changes of residence, thus inducing some degree of exposure misclassification. However, in a previous study in which we evaluated long-term historical residence of case and control children residing in Modena and Reggio Emilia municipalities [23], residential mobility was low; 70.3 % (225/320) of children examined in that investigation had never changed residence before the year of each case’s diagnosis; for children aged less 5 years, 82.2 % (152/185) had never changed residence (Malagoli et al., unpublished data). A further limitation was the exclusion of cases residing in mountainous areas owing to the inability of the CALINE4 model to handle rough topography.
When we limited the analysis to subjects younger than 5 years, the RRs associated with benzene exposure were higher than those calculated for the overall study population. The higher RR among these younger subjects could be explained in part by lower exposure misclassification, considering their lower probability of having changed addresses in the past, and by their likelihood of spending more time at home than older children; the percentage of children attending school in the 2003–2004 school year was 27 % and 25 % in the age group 0–3 years in Modena and Reggio Emilia provinces, respectively, rapidly increasing to 97 and 92 %, respectively, for age 5 (courtesy of Emanuela Bertozzi and Margherita Malagoli from the Reggio Emilia and the Modena Province Authorities). This higher RR is also compatible with the hypothesis that younger children have a greater susceptibility to adverse effects of benzene released by motor vehicles, not an entirely unexpected finding since some chemicals may pose a higher risk of cancer when exposure occurs during early life [34]. Several previous epidemiologic studies carried out age-specific subgroup analyses [9, 35–38]. Savitz and Feingold reported that excess risk associated with residential traffic density was limited to the 0–4 age group (RR = 5.6, 95 % CI 1.9–16.7), while the point estimate for RR for children aged 5–14 was below unity (RR = 0.4, 95 % CI 0.1–2.8) [35]; our results largely mirror these findings. Feychting et al. noted a higher overall childhood cancer relative risk associated with motor vehicle exhaust exposure (estimated by modeling nitrogen dioxide emissions) in the age group 0–5 compared with ages 5–9 and 10–14 [36]. On the other hand, Raaschou-Nielsen et al. [37] did not observe major age-related differences in childhood leukemia risk associated with benzene exposure. In addition, Reynolds et al. [38] did not identify an association between leukemia risk and road or traffic density in a study carried out on children younger than 5 years in California. In a further subgroup analysis for major childhood leukemia subtypes, i.e., acute lymphoblastic leukemia and acute myeloid leukemia, the relation with benzene exposure appeared considerably stronger for the latter category and in the youngest age group, although the estimates were imprecise owing to the small numbers involved. Such observation is of interest due to the already established association in adults with the same leukemia subtype, acute myeloid leukemia [4, 5, 13], but needs to be confirmed in much larger case series.
Previous epidemiologic studies generally based estimated individual exposure to pollutants from traffic on distance from main roads or data from air monitoring stations located in the same (generally broad) areas [4, 31]. These approaches are subject to substantial exposure misclassification, since exposure depends not only on distance but on the numbers and types of vehicles circulating on all nearby major and minor roads and meteorological conditions, data which were generally unavailable in previous studies. While our detailed exposure assessment is a strength, it must be noted that in our study, assessment of PM10 exposure was biased towards much lower values than actually measured in the study area since, unlike benzene, motorized traffic is not the major source of PM10 even in urban areas. In our investigation, estimated concentrations of PM10 at the 7 monitoring stations recording PM10 in the study area were 24.4 % of the measured values, a value very similar to that estimated as contribution of traffic to measured environmental levels of PM10 or its major component PM2.5 in recent studies in Milan (27.1 and 17–24 %, respectively) [39, 40].
Recent studies with a different design also suggested a relation between childhood leukemia and low-dose benzene exposure. One such study was an ecologic study in Texas based on census tract-specific benzene estimates [41]; others examined residence near petrol stations and automotive repair garages [42–45] or hazardous waste sites containing benzene [46]. Another study that used frequency of vehicle refueling by parents in the year before or during pregnancy and use of wood burners as indicators of benzene exposure did not find such association [47].
A distinctive feature of the present study was evaluation of PM10 as a potential confounder and an independent risk factor for leukemia, an association not analyzed so far to the best of our knowledge. PM10 concentrations may be considered a proxy for a number of contaminants hypothetically involved in the etiology of childhood leukemia and more generally of cancer, such as heavy metals and several volatile organic compounds including dioxins, benzene, benz(a)-pyrene, and 1-3 butadiene [18–21, 48, 49]. In our analyses, the independent association between PM10 and the disease was considerably weaker than for benzene but still possibly elevated, a relation that may be worth further study [48]. Mean annual levels of PM10 in the study area, as measured by the monitoring stations of the two provinces, were in several cases in the order of 40 μg/m3, a value which represents the EU standard [30] and is higher than the World Health Organization guideline of 20 μg/m3 [50].
The amount of exposure associated with increased childhood leukemia risk in the present study is not easy to determine, particularly since the induction period between benzene exposure and disease onset is unknown. In our study, we observed an excess risk mainly in the highest exposure categories (≥0.5 μg/m3 for average benzene concentration and ≥6 μg/m3 for maximum hourly benzene concentration). However, since levels of benzene decreased in the study area during the 12-year study period, and more generally in the nation as a whole, the critical amounts of benzene exposure might well have been those at the beginning of the period or immediately before it (estimated on the order of 5 μg/m3 or above), depending on the alleged length of the induction period [51, 52]. We note that the RR estimates were somewhat sensitive to the model specification (categorical versus continuous, untransformed versus log-transformed); however, the various modeling approaches were consistent in suggesting excess risk with higher exposure.
In conclusion, our study in this Italian community indicates that exposure to low environmental benzene concentrations released by motorized traffic may be a risk factor for childhood leukemia among children younger than 5 years of age, and particularly for acute myeloid leukemia, although this result could also reflect confounding from unmeasured traffic pollutants or other factors. Our findings offer limited evidence for a role of PM10.
References
Khalade A, Jaakkola MS, Pukkala E, Jaakkola JJ. Exposure to benzene at work and the risk of leukemia: a systematic review and meta-analysis. Environ Health. 2010;9:31.
Goldstein BD. Benzene as a cause of lymphoproliferative disorders. Chem Biol Interact. 2010;184:147–50.
Smith MT. Advances in understanding benzene health effects and susceptibility. Annu Rev Public Health. 2010;31:133–48.
Smith MT, Zhang L, McHale CM, Skibola CF, Rappaport SM. Benzene, the exposome and future investigations of leukemia etiology. Chem Biol Interact. 2011;192:155–9.
Cogliano VJ, Baan R, Straif K. Updating IARC’s carcinogenicity assessment of benzene. Am J Ind Med. 2011;54:165–7.
Wiemels J. Perspectives on the causes of childhood leukemia. Chem Biol Interact. 2012;196:59–67.
Glass DC, Gray CN, Jolley DJ, Gibbons C, Sim MR, Fritschi L, Adams GG, Bisby JA, Manuell R. Leukemia risk associated with low-level benzene exposure. Epidemiology. 2003;14:569–77.
Talbott EO, Xu X, Youk AO, Rager JR, Stragand JA, Malek AM. Risk of leukemia as a result of community exposure to gasoline vapors: a follow-up study. Environ Res. 2011;111:597–602.
Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, Contiero P, Bianchi N, Tagliabue G, Fissi R, Rossitto F, Berrino F. Childhood leukemia and road traffic: a population-based case-control study. Int J Cancer. 2004;108:596–9.
Visser O, van Wijnen JH, van Leeuwen FE. Residential traffic density and cancer incidence in Amsterdam, 1989–1997. Cancer Causes Control. 2004;15:331–9.
Goldstein BD, Liu Y, Wu F, Lioy P. Comparison of the effects of the US Clean Air Act and of smoking prevention and cessation efforts on the risk of acute myelogenous leukemia. Am J Public Health. 2011;101:2357–61.
Amigou A, Sermage-Faure C, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte G, Perel Y, Mechinaud F, Bordigoni P, Hemon D, Clavel J. Road traffic and childhood leukemia: the ESCALE study (SFCE). Environ Health Perspect. 2011;119:566–72.
McHale CM, Zhang L, Smith MT. Current understanding of the mechanism of benzene-induced leukemia in humans: implications for risk assessment. Carcinogenesis. 2012;33:240–52.
Langholz B, Ebi KL, Thomas DC, Peters JM, London SJ. Traffic density and the risk of childhood leukemia in a Los Angeles case-control study. Ann Epidemiol. 2002;12:482–7.
Zhou Y, Levy JI. Factors influencing the spatial extent of mobile source air pollution impacts: a meta-analysis. BMC Public Health. 2007;7:89.
Baldauf R, Thoma E, Hays M, Shores R, Kinsey J, Gullett B, Kimbrough S, Isakov V, Long T, Snow R, Khlystov A, Weinstein J, Chen FL, Seila R, Olson D, Gilmour I, Cho SH, Watkins N, Rowley P, Bang J. Traffic and meteorological impacts on near-road air quality: summary of methods and trends from the Raleigh near-road study. J Air Waste Manag Assoc. 2008;58:865–78.
Fujita EM, Campbell DE, Zielinska B, Arnott WP, Chow JC. Concentrations of air toxics in motor vehicle-dominated environments. Res Rep Health Eff Inst. 2011;156:3–77.
Sevastyanova O, Binkova B, Topinka J, Sram RJ, Kalina I, Popov T, Novakova Z, Farmer PB. In vitro genotoxicity of PAH mixtures and organic extract from urban air particles part II: human cell lines. Mutat Res. 2007;620:123–34.
Rodriguez JH, Pignata ML, Fangmeier A, Klumpp A. Accumulation of polycyclic aromatic hydrocarbons and trace elements in the bioindicator plants Tillandsia capillaris and Lolium multiflorum exposed at PM10 monitoring stations in Stuttgart (Germany). Chemosphere. 2010;80:208–15.
Pastuszka JS, Rogula-Kozlowska W, Zajusz-Zubek E. Characterization of PM10 and PM2.5 and associated heavy metals at the crossroads and urban background site in Zabrze, Upper Silesia, Poland, during the smog episodes. Environ Monit Assess. 2010;168:613–27.
Upadhyay N, Clements A, Fraser M, Herckes P. Chemical speciation of PM2.5 and PM10 in south Phoenix, AZ, USA. J Air Waste Manag Assoc. 2011;61:302–10.
Ferrari A, Dama E, Pession A, Rondelli R, Pascucci C, Locatelli F, Ferrari S, Mascarin M, Merletti F, Masera G, Arico M, Pastore G. Adolescents with cancer in Italy: entry into the national cooperative paediatric oncology group AIEOP trials. Eur J Cancer. 2009;45:328–34.
Malagoli C, Fabbi S, Teggi S, Calzari M, Poli M, Ballotti E, Notari B, Bruni M, Palazzi G, Paolucci P, Vinceti M. Risk of hematological malignancies associated with magnetic fields exposure from power lines: a case–control study in two municipalities of northern Italy. Environ Health. 2010;9:16.
Caline 4. A dispersion model for predicting air pollution concentration near roadways. Sacramento: State of California. Department of Transportation. Division of New Technology and Research; 1989.
Drufuca A, Battaiotto S, Bengo I, Rossi D, Torriani L. Variante Generale al Piano Territoriale di Coordinamento Provinciale. Procedura di simulazione della mobilità delle persone. Milano: Provincia di Modena–Polinomia; 2007.
Gandolfi P, Vittadini MR, Meggiato A, Gasparini G, Tupputi R, Montanari L, Torreggiani E, Ferrari S, Azzolini P, Torriani L, Drufuca A, Liguigli A, Dondè M, Mariotto A, Rondinella G. Piano della mobilità di area vasta di Reggio Emilia. Comune di Reggio Emilia. 2008. http://www.comune.re.it/retecivica/urp/retecivi.nsf/PESDocumentID/20E95864D1433C03C1257798003BDBDA?opendocument. Accessed 3 May 2012.
Gehrig R, Hill M, Buchmann B, Imhof D, Weingartner E, Baltensperger U. Separate determination of PM10 emission factors of road traffic for tailpipe emissions and emissions from abrasion and resuspension processes. Int J Environ Pollut. 2004;22:312–25.
Hastie TJ, Tibshirani RJ. Generalized additive models. Boca Raton: CRC Press; 1990.
R Development Core Team. A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2005.
Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off J Eur Union. 2008;L 152.
Pyatt D, Hays S. A review of the potential association between childhood leukemia and benzene. Chem Biol Interact. 2010;184:151–64.
Eden T. Aetiology of childhood leukaemia. Cancer Treat Rev. 2010;36:286–97.
Bonaventure A, Goujon-Bellec S, Rudant J, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Pasquet M, Michel G, Sirvent N, Bordigoni P, Ducassou S, Rialland X, Zelenika D, Hemon D, Clavel J. Maternal smoking during pregnancy, genetic polymorphisms of metabolic enzymes, and childhood acute leukemia: the ESCALE Study (SFCE). Cancer Causes Control. 2012;23:329–45.
Risk Assessment Guidance for Superfund. Volume I: Human health evaluation manual (Part F, Supplemental Guidance for Inhalation Risk Assessment). Washington: Office of Superfund Remediation and Technology Innovation, Environmental Protection Agency; 2009.
Savitz DA, Feingold L. Association of childhood cancer with residential traffic density. Scand J Work Environ Health. 1989;15:360–3.
Feychting M, Svensson D, Ahlbom A. Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health. 1998;24:8–11.
Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol. 2001;153:433–43.
Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A. Residential exposure to traffic in California and childhood cancer. Epidemiology. 2004;15:6–12.
Marcazzan GM, Ceriani M, Valli G, Vecchi R. Source apportionment of PM10 and PM2.5 in Milan (Italy) using receptor modelling. Sci Total Environ. 2003;317:137–47.
Perrone MG, Larsen BR, Ferrero L, Sangiorgi G, De Gennaro G, Udisti R, Zangrando R, Gambaro A, Bolzacchini E. Sources of high PM2.5 concentrations in Milan, Northern Italy: molecular marker data and CMB modelling. Sci Total Environ. 2012;414:343–55.
Whitworth KW, Symanski E, Coker AL. Childhood lymphohematopoietic cancer incidence and hazardous air pollutants in southeast Texas, 1995–2004. Environ Health Perspect. 2008;116:1576–80.
Harrison RM, Leung PL, Somervaille L, Smith R, Gilman E. Analysis of incidence of childhood cancer in the West Midlands of the United Kingdom in relation to proximity to main roads and petrol stations. Occup Environ Med. 1999;56:774–80.
Steffen C, Auclerc MF, Auvrignon A, Baruchel A, Kebaili K, Lambilliotte A, Leverger G, Sommelet D, Vilmer E, Hemon D, Clavel J. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons: a case-control study. Occup Environ Med. 2004;61:773–8.
Weng HH, Tsai SS, Chiu HF, Wu TN, Yang CY. Childhood leukemia and traffic air pollution in Taiwan: petrol station density as an indicator. J Toxicol Environ Health A. 2009;72:83–7.
Brosselin P, Rudant J, Orsi L, Leverger G, Baruchel A, Bertrand Y, Nelken B, Robert A, Michel G, Margueritte G, Perel Y, Mechinaud F, Bordigoni P, Hemon D, Clavel J. Acute childhood leukaemia and residence next to petrol stations and automotive repair garages: the ESCALE study (SFCE). Occup Environ Med. 2009;66:598–606.
Boberg E, Lessner L, Carpenter DO. The role of residence near hazardous waste sites containing benzene in the development of hematologic cancers in upstate New York. Int J Occup Med Environ Health. 2011;24:327–38.
Bailey HD, de Klerk NH, Fritschi L, Attia J, Daubenton JD, Armstrong BK, Milne E. Refuelling of vehicles, the use of wood burners and the risk of acute lymphoblastic leukaemia in childhood. Paediatr Perinat Epidemiol. 2011;25:528–39.
Knox EG. Childhood cancers and atmospheric carcinogens. J Epidemiol Community Health. 2005;59:101–5.
Ren Z, Zhang B, Lu P, Li C, Gao L, Zheng M. Characteristics of air pollution by polychlorinated dibenzo-p-dioxins and dibenzofurans in the typical industrial areas of Tangshan City, China. J Environ Sci. 2011;23:228–35.
WHO. Air quality guidelines for particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Global update 2005. Summary of risk assessment. Geneva: World Health Organization; 2006.
Rothman KJ, Greenland S, Lash TL. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 2008.
Triebig G. Implications of latency period between benzene exposure and development of leukemia—a synopsis of literature. Chem Biol Interact. 2010;184:26–9.
Acknowledgments
We gratefully acknowledge the cooperation of the Modena, the Emilia-Romagna and the national Directorates of the Italian Revenue Agency—Agenzia delle Entrate; Vittorio Colomba and Gianfrancesco Zanetti of the School of Law of the University of Modena and Reggio Emilia; Amelio Fraulini and Corrado Ugoletti of the Department of Urban Planning and GIS of Modena Province; Luisa Montanari, Paolo Azzolini, Stefano Cocchi and Simona Poli of the Reggio Emilia Municipality; Andrea Pession and Roberto Rondelli of the AIEOP Registry, Sant’Orsola Hospital, Bologna. Financial support to the study was provided by Associazione Sostegno Oncologia Ematologia Pediatrica—ASEOP and by the Department of the Environment of Reggio Emilia Municipality to Dr. Vinceti; by the US National Institute of Health to Dr. Crespi (grant NIH CA16042).
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Vinceti, M., Rothman, K.J., Crespi, C.M. et al. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case–control study in an Italian population. Eur J Epidemiol 27, 781–790 (2012). https://doi.org/10.1007/s10654-012-9727-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10654-012-9727-1