Abstract
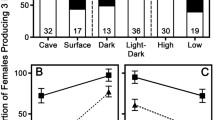
Life history traits within species often vary among different habitats. We measured female fecundity in mollies (Poecilia mexicana) from a H2S-rich cave and from a neighbouring surface habitat, as well as in laboratory-reared individuals of both populations raised in either light or continuous darkness. Compared to conspecifics from surface habitats, cave-dwelling P. mexicana had reduced fecundity (adjusted for size) in the field. In the laboratory, the fecundity of surface mollies was higher in light than in darkness, whereas fecundity in the cave mollies was almost unaffected by the ambient light conditions. Our results suggest a heritable component to the reduction in fecundity in female cave mollies. Moreover, the reduced plasticity in fecundity of cave mollies in response to light conditions might be an example of genetic assimilation or channelling of a life history trait in a population invading a new environment.
Similar content being viewed by others
References
Christiansen KA (1965) Behaviour and form in the evolution of cave Collembola. Evolution 19:529–532 doi:10.2307/2406249
Culver DC (1982) Cave life: evolution and ecology. Harvard University Press, Cambridge
Culver DC, Kane TC, Fong DW (1995) Adaptation and natural selection in caves: the evolution of Gammarus minus. Harvard University Press, Cambridge
de Jong G (1995) Phenotypic plasticity as a product of selection in a variable environment. Am Nat 145:493–512 doi:10.1086/285752
Gordon MS, Rosen DE (1962) A cavernicolous form of the poeciliid fish Poecilia sphenops from Tabasco, México. Copeia 1962:360–368 doi:10.2307/1440903
Howarth FG (1993) High-stress subterranean habitats and evolutionary change in cave-inhabiting arthropods. Am Nat 142:S65–S77 doi:10.1086/285523
Hüppop K (2000) How do cave animals cope with the food scarcity in caves? In: Wilkens H, Culver DC, Humphries WF (eds) Ecosystems of the world 30: subterranean ecosystems. Elsevier, Amsterdam, pp 159–188
Hüppop K, Wilkens H (1991) Bigger eggs in subterranean Astyanax fasciatus (Characidae, Pisces)—their significance and genetics. Z Zoolog Syst Evol Forsch 29:280–288
Langecker TG, Wilkens H, Parzefall J (1996) Studies on the trophic structure of an energy-rich Mexican cave (Cueva de las Sardinas) containing sulfurous water. Mem Biospeol 23:121–125
Parzefall J (2000) Tiefsee- und Höhlenbewohner im Vergleich. In: Uiblein F (ed) Tiefsee und Höhlen. Filander Verlag, Fürth D, pp 181–206
Parzefall J (2001) A review of morphological and behavioural changes in the cave molly, Poecilia mexicana, from Tabasco, Mexico. Environ Biol Fish 62:263–275 doi:10.1023/A:1011899817764
Pigliucci M (2005) Evolution of phenotypic plasticity: where are we going now? Trends Ecol Evol 20:481–486 doi:10.1016/j.tree.2005.06.001
Plath M, Parzefall J, Schlupp I (2003) The role of sexual harassment in cave and surface dwelling populations of the Atlantic molly, Poecilia mexicana (Poeciliidae, Teleostei). Behav Ecol Sociobiol 54:303–309 doi:10.1007/s00265-003-0625-0
Plath M, Hauswaldt JS, Moll K, Tobler M, García de León FJ, Schlupp I, Tiedemann R (2007a) Local adaptation and pronounced genetic differentiation in an extremophile fish, Poecilia mexicana, inhabiting a Mexican cave with toxic hydrogen sulfide. Mol Ecol 16:967–976 doi:10.1111/j.1365-294X.2006.03212.x
Plath M, Tobler M, Riesch R, Garcia de Léon FJ, Giere O, Schlupp I (2007b) Survival in an extreme habitat: the role of behavior and energy limitation. Naturwissenschaften 94:991–996 doi:10.1007/s00114-007-0279-2
Pouilly M, Miranda G (2003) Morphology and reproduction of the cavefish Trichomycterus chaberti and the related epigean Trichomycterus cf. barbouri. J Fish Biol 63:490–505 doi:10.1046/j.1095-8649.2003.00171.x
Poulson TL, Lavoie KH (2000) The trophic basis of subterranean ecosystems. In: Wilkens H, Culver DC, Humphries WF (eds) Ecosystems of the world 30: subterranean ecosystems. Elsevier, Amsterdam, pp 231–249
Reznick D, Endler JA (1982) The impact of predation on life history evolution in Trinidadian guppies (Poecilia reticulata). Evolution 36:160–177 doi:10.2307/2407978
Reznick D, Yang AP (1993) The influence of fluctuating resources on life history: patterns of allocation and plasticity in female guppies. Ecology 74:2011–2019 doi:10.2307/1940844
Reznick DN, Bryga HA, Endler JA (1990) Experimentally induced life-history evolution in a natural population. Nature 346:357–359 doi:10.1038/346357a0
Reznick DN, Butler MJ, Rodd H (2001) Life-history evolution in guppies. VII. The comparative ecology of high- and low-predation environments. Am Nat 157:126–140 doi:10.1086/318627
Roff DA (2002) Life history evolution. Sinauer Associates, Sunderland, MA
Romero A, Green SM (2005) The end of regressive evolution: examining and interpreting the evidence from cave fishes. J Fish Biol 67:3–32 doi:10.1111/j.0022-1112.2005.00776.x
Sibly RM, Calow P (1989) A life-cycle theory of responses to stress. Biol J Linn Soc Lond 37:101–116 doi:10.1111/j.1095-8312.1989.tb01908.x
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Tobler M (in press) Divergence in trophic ecology characterises colonisation of extreme habitats. Biol J Linn Soc Lond
Tobler M, Schlupp I, Heubel KU, Riesch R, García de León FJ, Giere O, Plath M (2006) Life on the edge: hydrogen sulfide and the fish communities of a Mexican cave and surrounding waters. Extremophiles 10:577–585 doi:10.1007/s00792-006-0531-2
Tobler M, Schlupp I, Garcia de Leon FJ, Glaubrecht M, Plath M (2007a) Extreme habitats as refuge from parasite infections? Evidence from an extremophile fish. Acta Oecol 31:270–275 doi:10.1016/j.actao.2006.12.002
Tobler M, Schlupp I, Plath M (2007b) Predation of cave fish (Poecilia mexicana, Poeciliidae) by a giant water-bug (Belostoma, Belostomatidae) in a Mexican sulfur cave. Ecol Entomol 32:492–495 doi:10.1111/j.1365-2311.2007.00892.x
Tobler M, Franssen CM, Plath M (2008) Male-biased predation on a cave fish by a giant water bug. Naturwissenschaften 95(8):775–779
Via S (1993) Phenotypic plasticity: target or by-product of selection in a variable environment? Am Nat 142:352–365 doi:10.1086/285542
Winemiller KO (1992) Life-history strategies and the effectiveness of sexual selection. Oikos 63:318–327 doi:10.2307/3545395
Acknowledgements
We are grateful to the people of Tapijulapa for their hospitality during our visits. L. D. Devenport, J. C. Kelly, E. C. Marsh-Matthews and L. J. Weider helped to improve previous drafts of the manuscript with their valuable comments. The Mexican Government kindly issued permits to conduct this research (Permiso de pesca de fomento numbers: 291002-613-1577, DGOPA/5864/260704/-2408 and DGOPA/16988/191205/-8101). This work represents partial fulfilment of the PhD requirements for R.R. Financial support came from the Deutsche Forschungsgemeinschaft (SCHL 344/15-1; PL 470/1-1, 2), the University of Oklahoma, the German Ichthyological Association (to M.T. and M.P) as well as the Basler Foundation for Biological Research, the Janggen-Poehn-Foundation, the Roche Research Foundation, and the Wolfermann-Nägeli-Foundation (to M.T.). For the collection of these data, the authors have adhered to the Guidelines for the Use of Animals in Research. The experiments reported here are in agreement with the respective laws in Germany.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riesch, R., Tobler, M., Plath, M. et al. Offspring number in a livebearing fish (Poecilia mexicana, Poeciliidae): reduced fecundity and reduced plasticity in a population of cave mollies. Environ Biol Fish 84, 89–94 (2009). https://doi.org/10.1007/s10641-008-9392-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10641-008-9392-0