Abstract
Purpose
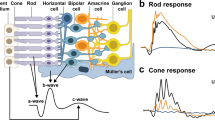
To examine changes in inner retinal function of nob2 mice, expressing a null mutation in Cacna1f encoding the CaV1.4 subunit of voltage-dependent calcium channels. CACNA1F mutations underlie one form of incomplete X-linked congenital stationary night blindness (CSNB2). In addition to a loss of dark-adapted (rod-driven) visual sensitivity, electroretinogram (ERG) b-waves and oscillatory potentials (OPs) are decreased in CSNB2 patients.
Methods
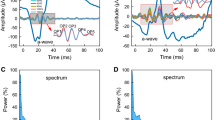
ERGs were recorded under dark-and light-adapted conditions from the corneal surface of nob2 mice, WT littermates and nob4 mice. ERG frequency spectra were calculated by fast Fourier transform (FFT). A FFT-based high-pass filter was used to derive OP waveforms.
Results
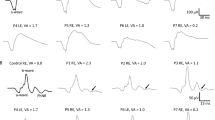
Under dark-adapted conditions, the dominant frequency of the OPs varied between 90 to 120 Hz in WT mice. In WT mice, OP frequency first increased with flash intensity and then decreased at the highest flash levels while overall OP amplitude increased monotonically with increasing flash intensity. In response to low stimulus flashes, reliable OPs were not obtained from nob2 mice. OPs were only seen at stimulus intensities at or above −1.8 log cd s/m2, where they occurred at a lower frequency range (70–90 Hz) than for WT mice. When flash stimuli were superimposed against a steady rod-desensitizing adapting field, the amplitude and frequency of WT OPs increased with flash intensity above 0.4 log cd s/m2. In comparison to WT results, cone-mediated OPs obtained from nob2 mice were smaller in amplitude, of lower frequency and had delayed implicit times. We compared the extent to which OPs and the b-wave were reduced in nob2 mice, by normalizing to the results obtained from WT mice. In comparison to the b-wave, the OPs were relatively spared, under both dark- and light-adapted conditions.
Conclusions
In nob2 mice, rod- and cone-driven OPs are reduced in amplitude and occur at a lower frequency range. Since CaV1.4 is expressed in both the inner and outer plexiform layers, these changes are likely to reflect reduced transmission from photoreceptors to bipolar cells as well as alterations in inner retinal function. That the OPs were better preserved than b-waves suggests that inner retinal pathways may be reorganized in response to the decreased bipolar cell response in nob2 mice.
















Similar content being viewed by others
References
Copenhagen DR, Jahr CE (1989) Release of endogenous excitatory amino acids from turtle photoreceptors. Nature 341:536–539
Schmitz Y, Witkovsky P (1997) Dependence of photoreceptor glutamate release on a dihydropyridine-sensitive calcium channel. Neurosci 78:1209–1216
Bech-Hansen NT, Naylor MJ, Maybaum TA et al (1998) Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nature Genet 19:264–267
Strom TM, Nyakatura G, Apfelstedt-Sylla E et al (1998) An L-type calcium-channel gene mutated in incomplete X-linked congenital stationary night blindness. Nature Genet 19:260–263
Boycott KM, Maybaum TA, Naylor MJ et al (2001) A summary of 20 CACNA1F mutations identified in 36 families with incomplete X-linked congenital stationary night blindness, and characterization of splice variants. Hum Genet 108:91–97
Wutz K, Sauer C, Zrenner E et al (2002) Thirty distinct CACNA1F mutations in 33 families with incomplete type of XLCSNB and Cacna1f expression profiling in mouse retina. Eur J Hum Genet 10:449–456
Wycisk KA, Zeitz C, Feil S et al (2006) Mutation in the auxiliary calcium-channel subunit CACNA2D4 causes autosomal recessive cone dystrophy. Am J Hum Genet 79:973–977
Zeitz C, Kloeckener-Gruissem B, Forster U et al (2006) Mutations in CABP4, the gene encoding the Ca2+-binding protein 4, cause autosomal recessive night blindness. Am J Hum Genet 79:657–667
Miyake Y, Yagasaki K, Horiguchi M et al (1986) Congenital stationary night blindness with negative electroretinogram. A new classification. Arch Ophthalmol 104:1013–1020
Mansergh F, Orton NC, Vessey JP et al (2005) Mutation of the calcium channel gene Cacna1f disrupts calcium signaling, synaptic transmission and cellular organization in mouse retina. Hum Mol Genet 14:3035–3046
Chang B, Heckenlively JR, Bayley PR et al (2006) The nob2 mouse, a null mutation in Cacna1f: anatomical and functional abnormalities in the outer retina and their consequences on ganglion cell visual responses. Vis Neurosci 23:11–24
Pinto LH, Vitaterna MH, Shimomura K et al (2007) Generation, identification and functional characterization of the nob4 mutation of Grm6 in the mouse. Vis Neurosci 24:111–123
Ruether K, Grosse J, Matthiessen E et al (2000) Abnormalities of the photoreceptor-bipolar cell synapse in a substrain of C57BL/10 mice. Invest Ophthalmol Vis Sci 41:4039–4047
Wycisk KA, Budde B, Feil S et al (2006) Structural and functional abnormalities of retinal ribbon synapses due to Cacna2d4 mutation. Invest Ophthalmol Vis Sci 47:3523–3530
Haeseleer F, Imanishi Y, Maeda T et al (2004) Essential role of Ca2+-binding protein 4, a Cav1.4 channel regulator, in photoreceptor synaptic function. Nature Neurosci 7:1079–1087
Morgans CW (2001) Localization of the α1F calcium channel subunit in the rat retina. Invest Ophthalmol Vis Sci 42:2414–2418
Ball SL, Powers PA, Shin HS et al (2002) Role of the β2 subunit of voltage-dependent calcium channels in the retinal outer plexiform layer. Invest Ophthalmol Vis Sci 43:1595–1603
Ridder W 3rd, Nusinowitz S, Heckenlively JR (2002) Causes of cataract development in anesthetized mice. Exp Eye Res 75:365–370
Peachey NS, Goto Y, al-Ubaidi MR et al (1993) Properties of the mouse cone-mediated electroretinogram during light adaptation. Neurosci Lett 162:9–11
Lei B, Yao G, Zhang K et al (2006) Study of rod-and cone-driven oscillatory potentials in mice. Invest Ophthalmol Vis Sci 47:2732–2738
Gur M, Zeevi Y (1980) Frequency-domain analysis of the human electroretinogram. J Opt Soc Amer 70:53–59
Wachtmeister L (1998) Oscillatory potentials in the retina: what do they reveal? Prog Retin Eye Res 17:485–521
Dong CJ, Agey P, Hare WA (2004) Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci 21:533–543
Gutierrez O, Spiguel RD (1973) Oscillatory potentials of the cat retina: effects of adrenergic drugs. Life Sci 13:991–999
Wachtmeister L, Dowling JE (1978) The oscillatory potentials of the mudpuppy retina. Invest Ophthalmol Vis Sci 17:1176–1188
Wachtmeister L (1980) Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG) I. GABA-and glycine antagonists. Acta Ophthalmol (Copenh) 58:712–725
Wachtmeister L (1981) Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG). II. Glutamate-aspartate-and dopamine antagonists. Acta Ophthalmol (Copenh) 59:247–258
Wachtmeister L (1981) Further studies of the chemical sensitivity of the oscillatory potentials of the electroretinogram (ERG). III. Some omega amino acids and ethanol. Acta Ophthalmol (Copenh) 59:609–619
Wachtmeister L (1983) The action of opiates on the oscillatory potentials of the mudpuppy retina. Doc Ophthalmol Proc Series 37:317–323
Kojima M, Zrenner E (1978) Off-components in response to brief light flashes in the oscillatory potential of the human electroretinogram. Albrecht Von Graefes Arch Klin Exp Ophthalmol 206:107–120
Alcayaga J, Bustamante S, Gutierrez OC (1989) Fast activity and oscillatory potential of carp retina in the frequency domain. Vision Res 29:949–955
Lachapelle P (1991) The effect of a slow flicker on the human photopic oscillatory potentials. Vision Res 31:1851–1857
Peachey NS, Alexander KR, Derlacki DJ et al (1991) Effects of light adaptation on the response characteristics of human oscillatory potentials. Electroencephalogr Clin Neurophysiol 78:27–34
Heckenlively JR, Martin DA, Rosenbaum AL (1983) Loss of electroretinographic oscillatory potentials, optic atrophy, and dysplasia in congenital stationary night blindness. Am J Ophthalmol 96:526–534
Lachapelle P, Little JM, Polomeno RC (1983) The photopic electroretinogram in congenital stationary night blindness with myopia. Invest Ophthalmol Vis Sci 24:442–450
Lachapelle P, Rousseau S, McKerral M et al (1998) Evidence supportive of a functional discrimination between photopic oscillatory potentials as revealed with cone and rod mediated retinopathies. Doc Ophthalmol 95:35–54
Berson EL, Sandberg MA, Maguire A et al (1986) Electroretinograms in carriers of blue cone monochromatism. Am J Ophthalmol 102:254–261
Acknowledgments
This research was supported by the NEI (R01 EY14465; R24 EY15638), the Department of Veterans Affairs, a Challenge grant from Research to Prevent Blindness to the Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, and a State of Ohio BRTT grant. The authors are grateful to Jiang Wu for care of experimental mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, M., Peachey, N.S. Attenuation of oscillatory potentials in nob2 mice. Doc Ophthalmol 115, 173–186 (2007). https://doi.org/10.1007/s10633-007-9058-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-007-9058-9