Abstract
Background and Aims
The anti-inflammatory and reparative properties of mesenchymal stem cells (MSCs) make them a promising tool for treating immune-mediated and inflammatory disorders. However, whether MSCs can be used for treatment of inflammatory bowel disease (IBD) still remains unclear. In this study, a dextran sulfate sodium (DSS)-induced mouse colitis model was used to test the hypothesis that infused bone marrow-derived MSCs could exert anti-inflammatory effects against experimental colitis.
Methods
DSS-induced colitis mice were injected with 1 × 106 MSCs [in phosphate-buffered saline (PBS)] via the tail vein. Control colitis mice received PBS alone. To trace the injected cells in vivo, MSCs were labeled with chloromethyl-benzamidodialkylcarbocyanine (CM-DiI). On day 15 of the experiment, the colon was sectioned and examined for histopathological changes. Pro-inflammatory cytokines [tumor necrosis factor-alpha (TNF-α), interleukin (IL)-1β] in the inflamed colon were analyzed by real-time reverse-transcription polymerase chain reaction (RT-PCR). Serum values of TNF-α in mice were evaluated quantitatively by enzyme-linked immunosorbent assay (ELISA) analysis.
Results
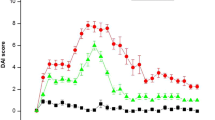
DSS-induced colitis showed symptoms similar to ulcerative colitis in humans, including body weight loss, bloody diarrhea, mucosal ulceration, and shortening of the colon. Bone marrow-derived MSCs significantly ameliorated the clinical and histopathologic severity of DSS colitis compared with non-MSC control. Pro-inflammatory cytokines in both the inflamed colon (TNF-α, IL-1β) and serum (TNF-α) were downregulated in MSC-treated mice in contrast to control. CM-DiI-labeled MSCs accumulated in inflamed regions of the colon, mainly in the submucosa.
Conclusions
Systemic infusion of bone marrow-derived MSCs may exert therapeutic efficacy on acute DSS-induced colitis in mice through their anti-inflammatory effects, which demonstrates the feasibility of using bone marrow-derived MSCs to treat IBD.








Similar content being viewed by others
References
Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–1640.
Xavier RJ, Podilsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434.
Kucharzik T, Maaser C, Lügering A, et al. Recent understanding of IBD pathogenesis: implications for future therapies. Inflamm Bowel Dis. 2006;12:1068–1083.
Potian JA, Aviv H, Ponzio NM, Harrison JS, Rameshwar P. Veto-like activity of mesenchymal stem cells: functional discrimination between cellular responses to alloantigens and recall antigens. J Immunol. 2003;171:3426–3434.
Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringdén O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896.
Bianco P, Robey PG, Simmons PJ. Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2008;2:313–319.
Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347.
Jones E, McGonagle D. Human bone marrow mesenchymal stem cells in vivo. Rheumatology (Oxford). 2008;47:126–131.
Brooke G, Cook M, Blair C, et al. Therapeutic applications of mesenchymal stromal cells. Semin Cell Dev Biol. 2007;18:846–858.
Păunescu V, Deak E, Herman D, et al. In vitro differentiation of human mesenchymal stem cells to epithelial lineage. J Cell Mol Med. 2007;11:502–508.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736.
Fibbe WE, Nauta AJ, Roelofs H. Modulation of immune responses by mesenchymal stem cells. Ann N Y Acad Sci. 2007;1106:272–278.
Noël D, Djouad F, Bouffi C, et al. Multipotent mesenchymal stromal cells and immune tolerance. Leuk Lymphoma. 2007;48:1283–1289.
Zhang B, Liu R, Shi D, et al. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46–57.
Rinden O, Uzunel M, Rassmuson I, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006;81:1390–1397.
Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441.
Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus host disease: a phase II study. Lancet. 2008;371:1579–1586.
Wirtz S, Neufert C, Weigmann B. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546.
Naito Y, Takagi T, Kuroda M, et al. An orally active matrix metalloproteinase inhibitor, ONO-4817, reduces dextran sulfate sodium induced colitis in mice. Inflamm Res. 2004;53:462–468.
Obermeier F, Kojouharoff G, Hans W, et al. Interferon-gamma (IFN-gamma) and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin Exp Immunol. 1999;116:238–245.
Ogata H, Hibi T. Cytokine and anti-cytokine therapies for inflammatory bowel disease. Curr Pharm Des. 2003;9:1107–1113.
Gonzalez MA, Gonzalez-Rey E, Rico L, et al. Treatment of experimental arthritis by inducing immune tolerance with human adipose derived mesenchymal stem cells. Arthr Rheum. 2009;60:1006–1019.
Prockop DJ. Repair of tissues by adult stem/progenitor cells (MSCs): controversies, myths, and changing paradigms. Mol Ther. 2009;17:939–946.
Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stemcells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761.
Prevosto C, Zancolli M, Canevali P, et al. Generation of CD4+ or CD8+ regulatory T cells upon mesenchymal stem cell-lymphocyte interaction. Haematologica. 2007;92:881–888.
Kong QF, Sun B, Bai SS, et al. Administration of bone marrow stromal cells ameliorates experimental autoimmune myasthenia gravis by altering the balance of Th1/Th2/Th17/Treg cell subsets through the secretion of TGF-beta. J Neuroimmunol. 2009;207:83–91.
Phinney D, Prockop D. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdiffererentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902.
Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109–116.
Komori M, Tsuji S, Tsujii M, et al. Involvement of bone marrow-derived cells in healing of experimental colitis in rats. Wound Repair Regen. 2005;13:109–118.
Brittan M, Chance V, Elia G, et al. A regenerative role for bone marrow following experimental colitis: contribution to neovasculogenesis and myofibroblasts. Gastroenterology. 2005;128:1984–1995.
Khalil PN, Weiler V, Nelson PJ, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954.
Bernardo ME, Zaffaroni N, Novara F, et al. Human bone marrow derived mesenchymal stem cells do not undergo transformation after long-term in vitro culture and do not exhibit telomere maintenance mechanisms. Cancer Res. 2007;67:9142–9149.
Djouad F, Plence P, Bony C, et al. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837–3844.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 30872461) and Guangdong Natural Science Foundation (no. 8151008901000107).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xiao-Wen He and Xiao-Sheng He contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, XW., He, XS., Lian, L. et al. Systemic Infusion of Bone Marrow-Derived Mesenchymal Stem Cells for Treatment of Experimental Colitis in Mice. Dig Dis Sci 57, 3136–3144 (2012). https://doi.org/10.1007/s10620-012-2290-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2290-5