Abstract
Purpose
The FANCM c.5101C>T nonsense mutation was previously found to associate with breast cancer in the Finnish population, especially among triple-negative cases. Here, we studied the prevalence of three other FANCM variants: c.5791C>T, which has been reported to predispose to familial breast cancer, and the c.4025_4026delCT and c.5293dupA variants recently identified in Finnish cancer patients.
Methods
We genotyped the FANCM c.5791C>T mutation in 4806 invasive breast cancer patients, including BRCA1/2 mutation negative familial cases and unselected cases, and in 2734 healthy population controls from four different geographical areas of Finland. The association of the mutation with breast cancer risk among patient subgroups was statistically evaluated. We further analyzed the combined risk associated with c.5101C>T and c.5791C>T mutations. We also genotyped 526 unselected ovarian cancer patients for the c.5791C>T mutation and 862 familial breast cancer patients for the c.4025_4026delCT and c.5293dupA variants.
Results
The frequency of the FANCM c.5791C>T mutation was higher among breast cancer cases than in controls (OR 1.94, 95% CI 0.87–4.32, P = 0.11), with a statistically significant association with triple-negative breast cancer (OR 5.14, 95% CI 1.65–16.0, P = 0.005). The combined analysis for c.5101C>T and c.5791C>T carriers confirmed a strong association with breast cancer (OR 1.86, 95% CI 1.32–2.49, P = 0.0002), especially among the triple-negative patients (OR 3.08, 95% CI 1.77–5.35, P = 0.00007). For the other variants, only one additional c.4025_4026delCT carrier and no c.5293dupA carriers were observed.
Conclusions
These results support the role of FANCM as a breast cancer susceptibility gene, particularly for triple-negative breast cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fanconi Anemia complementation group M (FANCM) is a multifunctional protein, interacting with several partners to activate the Fanconi anemia (FA) repair pathway. The pathway includes 19 associated proteins, which form multiprotein complexes to repair damaged DNA, especially stalled replication forks induced by interstrand cross-linking (ICL) agents [1, 2]. FA itself is a rare, recessively inherited genetic disorder causing congenital defects, hematologic abnormalities, and cancer predisposition [1].
Despite the nomenclature, FANCM does not appear to have a role in the FA disease, as the initial case, which led to the FANCM association with FA, also carried biallelic FANCA mutations. Furthermore, homozygous loss-of-function mutations in FANCM have been identified in individuals without any FA symptoms [3, 4]. However, FANCM has an undeniable role in the FA pathway, as it recruits other DNA damage response proteins to the sites of DNA lesions [4]. Several FA genes (FANCD1/BRCA2, FANCN/PALB2, FANCJ/BRIP1, FANCO/RAD51C, and FANCS/BRCA1) [5,6,7,8,9,10,11] are high- or moderate-risk breast or ovarian cancer susceptibility genes and previous studies have connected also heterozygous FANCM mutations with breast cancer predisposition [12,13,14].
We previously identified the FANCM c.5101C>T nonsense mutation (rs147021911, p.Gln1701*) in exon 20 by exome sequencing of germline DNA samples from 24 BRCA1/2-negative breast cancer patients and further genotyped it in a large series of Finnish breast cancer patients and healthy population controls [12]. The mutation was found to be associated with breast cancer with an odds ratio (OR) of 1.86 [95% confidence interval (CI) 1.26–2.75, P = 0.0018], especially among triple-negative breast cancer [TNBC; estrogen (ER) and progesterone (PR) receptor and HER2 negative] cases (OR 3.56, 95% CI 1.81–6.98, P = 0.0002). According to the ExAC [15] database this mutation is more common in Finland (carrier frequency ~1.8%) than in other European populations (carrier frequency ~0.3%).
Another, yet more rare FANCM mutation, c.5791C>T (rs144567652, p.Arg1931*) in exon 22, has been identified with exome sequencing of breast cancer patients [16]. Further analysis of familial breast cancer cases and healthy controls from several populations showed an association between the mutation and familial breast cancer (OR 3.93, 95% CI 1.28–12.11, P = 0.017) [13]. A recent study in a German population produced similar results, where the loss-of-function mutations in the FANCM gene were associated with both familial breast cancer and TNBC [14].
c.5791C>T mutation has also been identified in two colorectal cancer patients [17], and since FANCM is functionally connected with the mismatch repair genes MSH2/MSH6, it has previously been considered as a potential candidate gene for hereditary non-polyposis colorectal cancer (HNPCC) [17, 18]. However, a combined analysis of larger datasets did not reveal statistically significant association between FANCM mutations and the disease [19].
Here, we have investigated the association of the FANCM c.5791C>T mutation with breast cancer risk in familial and unselected breast cancer cases among 4806 invasive breast cancer patients and 2734 healthy population controls from four different geographical areas of Finland. We further evaluated the breast cancer risk by subgroups of patients as well as ovarian cancer risk among 526 ovarian cancer patients. Also, the recently identified c.4025_4026delCT (p.Ser1342*) and c.5293dupA (p.Thr1765Asnfs*3) variants in the FANCM gene were studied among 862 familial breast cancer patients from the Helsinki area.
Moreover, to further study the risk associated with truncating C-terminal FANCM mutations, we analyzed the current results of the c.5791C>T mutation and risk estimates of the c.5101C>T mutation, including the previously published results from Helsinki and Tampere area [12] and genotyping results from Oulu and Kuopio.
Methods
Subjects
Helsinki breast cancer series
The FANCM c.5791C>T mutation was genotyped in 2391 breast cancer cases (including unselected and familial patients) and 1258 healthy female population controls from the Helsinki area. Unselected breast cancer patient samples were collected at the Helsinki University Central Hospital in two phases. During 1997–1998 and 2000, 884 samples were collected at the Department of Oncology. These samples include 79% of all consecutive, newly diagnosed breast cancer cases during the collection periods [20, 21]. During 2001–2004, 986 samples, including 87% of all consecutive, newly diagnosed breast cancer cases were collected at the Department of Surgery [21]. Only invasive cases were included in this study.
The familial cases were collected at the Helsinki University Central Hospital Departments of Oncology and Clinical Genetics [22, 23]. Of these, 523 patients had a strong family history with at least three breast or ovarian cancers among first- or second-degree relatives (including the proband), and 555 patients had at least one first-degree relative affected with breast or ovarian cancer. All the familial cohort patients with at least three breast or ovarian cancers among first- or second-degree relatives were tested negative for BRCA1/2 mutations and the patients with one affected relative were tested negative for the Finnish BRCA1/2 founder mutations as previously described [24,25,26]. Eight hundred and sixty two familial cases were genotyped for the c.4025_4026delCT and c.5293dupA variants and 1078 familial cases were genotyped for the FANCM c.5791C>T mutation.
The samples are genomic DNA isolated from peripheral blood. The patient genealogies were confirmed from the population registries or hospital records and cancer diagnoses from the hospital records or the Finnish Cancer Registry. Hormone receptor status was collected from pathology reports as described earlier [27]. The 1258 genotyped population controls were healthy female blood donors from the Helsinki area.
The unselected ovarian cancer cohort was collected at the Helsinki University Central Hospital Department of Obstetrics and Gynecology in 1998 as previously described [28]. Blood samples were collected from invasive epithelial ovarian carcinoma treated patients during routine follow-up visits to the clinic. Additional samples were also collected between 1998 and 2006. Out of the 526 independent samples included in the analysis in this study, 408 were genomic DNA isolated from blood and 118 samples were tumor-DNA. Of the genomic samples, 171 were serous, 64 mucinous, and 43 endometrioid subtype. 129 samples were other ovarian cancer subtypes, for one sample the subtype is not known. Out of the tumor samples, 104 were serous and two were other subtypes, 12 samples were of unknown subtype.
Tampere breast cancer series
The unselected breast cancer patient sample set from the Tampere area was collected at the Tampere University Hospital as previously described [20, 22]. Additional 336 incident cases were collected in 1996–2004 at the Tampere University Hospital. Two hundred and forty nine cases had also familial background. Hormone receptor status information was obtained from patient pathology reports. Only invasive cases were included in this study. All samples are genomic DNA isolated from peripheral blood. The control cohort for the Tampere dataset consists of 808 healthy female blood donors from the Tampere area.
Kuopio breast cancer series
430 patients with invasive breast cancer from the Kuopio area were genotyped for the FANCM c.5791C>T and c.5101C>T mutations. These patients belong to prospective population-based case–control study, The Kuopio Breast Cancer Project (KBCP), which was conducted in between 1990 and 1996. Samples were collected from women entering the Kuopio University Hospital due to breast symptoms and who were eventually diagnosed as having breast cancer. All samples are genomic DNA isolated from peripheral blood. Information about hormonal receptor status was obtained from hospital registries [29, 30]. The control cohort consists of 158 healthy blood donors from the Kuopio area.
Oulu breast cancer series
The FANCM c.5791C>T and c.5101C>T mutations were screened among 1323 breast cancer cases (1147 unselected, 153 familial, and 56 young breast cancer patients) and 510 healthy female controls from the Oulu area. The unselected breast cancer samples were collected from patients operated at the Oulu University Hospital during 2000–2014, and they were unselected for age at disease onset and a family history of cancer. Only invasive cases were included in this study. The familial breast cancer cases were affected index individuals of Northern Finnish breast or breast and ovarian cancer families, and the young cohort consisted of breast cancer patients unselected for family history of cancer but with early disease onset (≤40 years), which suggests a plausible hereditary predisposition regardless of the family history [31,32,33]. Only BRCA1/2 mutation negative familial and young cases were included in this study. Hormone receptor status was collected from pathology reports as described earlier [34]. The control cohort consisted of 510 healthy female blood donors from the Oulu area. All samples were genomic DNA isolated from peripheral blood.
This study was performed with informed consent from all the patients and permission from the ethics committees of University of Helsinki, Tampere University Hospital, Oulu University Hospital, University of Eastern Finland, and Kuopio University Hospital Board on Research Ethics.
Identification of the mutations
The FANCM c.5791C>T mutation has been found to associate with familial breast cancer and TNBC [13, 14]. It has also been reported in the Finnish population as an enriched loss-of-function mutation [3] and identified in gene panel sequencing of Finnish familial breast cancer patients [35].
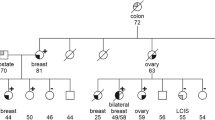
The c.4025_4026delCT variant was identified at Lund University in one patient from 100 Finnish high-risk breast cancer cases with panel sequencing, in which also two carriers of the previously published c.5101C>T mutation were identified. SureselectXT Custom 3-5.9 Mb library kit (Agilent Technology) was used to capture DNA fragments from the target genes. Sequencing was performed on the Illumina HiSeq 2500 with 2 × 94 to 2 × 101 bp paired-end reads.
The c.5293dupA variant was identified by exome sequencing of genomic DNA samples from 44 Finnish cancer patients with familial history of breast cancer. Exome sequencing was executed at the Genome Quebec Innovation Centre. To capture the exomic regions, Roche Nimblegen SeqCap EZ Exome v3 kit was used. The sequencing was performed on Illumina HiSeq 2000 sequencer with 100 bp paired-end reads.
The c.5101C>T mutation was identified by exome sequencing as previously described [12].
Genotyping
Genotyping of the FANCM c.5791C>T mutation for the Helsinki and Tampere sample sets was performed with Sequenom MassARRAY system using iPLEX Gold assays (Sequenom) at FIMM (University of Helsinki). Variants c.4025_4026delCT and c.5293dupA were genotyped with TaqMan real-time PCR. 7500 Fast Real Time system was utilized by using TaqMan SNP Genotyping Custom assays and TaqMan Genotyping MasterMix (Applied Biosystems). Genotype calling was performed with 7500 RealTime PCR System and 7500 software (version 2.06, Applied Biosystems). Genotyping for the FANCM c.5101C>T mutation was performed as previously described [12]. Positive controls were used in all analyses and all mutations were confirmed with Sanger sequencing. It is to be noted that one patient from the Helsinki dataset (counted as one in the analysis) carries both c.5101C>T and c.5791C>T mutations. Unfortunately, we were not able to determine whether the mutations are in cis or in trans; however, if they were in cis, this genotype would be extremely rare.
FANCM c.5791C>T and c.5101C>T screening for Oulu and Kuopio sample sets was performed using High Resolution Melt analysis (CFX96, Bio-Rad) with Type-it HRM reagents (Qiagen). Positive control DNA was included in all analyses, and samples with positive-like or differing melting curves were validated by Sanger sequencing (ABI3130xl, Applied Biosystems).
Statistical analyses
All statistical analyses were performed using R (version 3.02) statistical software (http://www.r-project.org) or IBM SPSS Statistics for Windows, version 22.0. For the risk analyses, two-sided P-values were calculated using Pearson´s χ 2-test or Fisher´s exact test if the expected number of cell count was five or less. P < 0.05 was considered statistically significant. Betas and standard errors of the different studies were combined in “rmeta”-package to examine heterogeneity between studies. In heterogeneity analysis, P < 0.10 was considered statistically significant.
In the combined analyses, all the datasets were pooled and the odds ratios and P-values were estimated with logistic regression model stratified by study. Separate analysis were conducted for the subgroups defined by histopathology and family history of the disease. In the combined analysis including datasets for both FANCM c.5791C>T and c.5101C>T mutations, patients with genotyping results for both mutations were included in the analysis.
Results
Genotyping of the FANCM c.5791C>T mutation in the case–control sample sets
The FANCM c.5791C>T mutation was identified in altogether 28 breast cancer patients and eight controls among 4806 breast cancer patients and 2734 population controls from four different geographical areas of Finland (Helsinki, Tampere, Kuopio, and Oulu). The population frequency was highest in Northern and Eastern Finland (Oulu 0.6% and Kuopio 0.6%) and lowest in Southern and Southwestern Finland (Helsinki 0.2% and Tampere 0.1%). Among the BRCA1/2-negative familial patients from Helsinki and Oulu datasets (N = 1231), eight mutation carriers were identified, and among the 526 ovarian cancer patients from Helsinki, two mutation carriers were identified (Table 1).
We evaluated the breast cancer risk among all genotyped patients (all BC) in each dataset separately (Table 1). Furthermore, genotyped patients were divided into subgroups according to family history of cancer, ER status, and triple-negative subtype to study the risks by breast cancer phenotypes.
Breast cancer risk was increased among all breast cancer patients in the Helsinki (OR 2.11, 95% CI 0.59–7.49, P = 0.24), Tampere (OR 6.14, 95% CI 0.72–52.70, P = 0.10), and Oulu datasets (OR 1.16, 95% CI 0.31–4.30, P = 1), albeit with no statistically significant P-values. In the Kuopio dataset, only two mutations carriers were identified (OR 0.73, 95% CI 0.07–8.15, P = 1) (Table 1). No significant heterogeneity was seen between the studies (P = 0.9).
However, the FANCM c.5791C>T mutation associated significantly with TNBC in the Helsinki dataset (OR 9.09, 95% CI 1.82–45.49, P = 0.02). In the Tampere and Oulu datasets, two additional triple-negative cases were identified.
The analysis of ovarian cancer cases from the Helsinki dataset suggested possibly slightly increased risk among c.5791C>T carriers (OR 1.60, 95% CI 0.27–9.58, P = 0.64, Table 1); however, there were only two mutation carriers identified. One patient has serous ovarian cancer, whereas the other mutation carrier has been diagnosed with mucinous subtype of the disease.
We further performed a combined analysis of the four studies to evaluate the risk among all studied breast cancer patients. An elevated breast cancer risk for the c.5791C>T carriers was seen among all breast cancer patients (OR 1.94, 95% CI 0.87–4.32, P = 0.11), and particularly in the TNBC subgroup (OR 5.14, 95% CI 1.65–16.0), with a statistically significant P value (0.005) (Table 2).
In addition, the breast cancer risk was increased also in the other subgroups, e.g., ER negative (OR 2.34, 95% CI 0.75–7.35, P = 0.14) and familial breast cancer (OR 2.50, 95% CI 0.83–7.51, P = 0.10) (Table 2), but the results did not reach statistical significance.
Combined analyses of the FANCM c.5791C>T and c.5101C>T mutations
We combined the results from the current analyses of the FANCM c.5791C>T mutation with the previous risk study of the FANCM c.5101C>T mutation in the Helsinki and Tampere datasets [12], and genotyping results from Oulu and Kuopio cases and controls. Highly significant association was seen between breast cancer and carrying either of the mutations (OR 1.86, 95% CI 1.32–2.49, P = 0.0002). The risk was consistently increased in all subgroups of patients, with highest risk and the most significant association seen among the triple-negative patients (OR 3.08, 95% CI 1.77–5.35, P = 0.00007) (Table 3). No heterogeneity was seen between mutations (P = 0.7). All analyses were stratified by study.
Genotyping of the FANCM c.4025_4026delCT and c.5293dupA variants
The FANCM c.5293dupA and c.4025_4026delCT variants were genotyped among 862 familial breast cancer patients from the Helsinki area. No additional mutation carriers were identified for c.5293dupA variant which may represent a unique mutation in the family where it was identified. The mutation was originally identified in exome sequencing of a uterine cancer patient with a family history of breast cancer.
One additional carrier was identified for c.4025_4026delCT, totaling two carriers for this rare variant and was not studied further. The patients´ ages at diagnosis were 42 and 54, respectively. One carrier had ER-negative breast cancer, whereas the other had ER-positive disease. Both had family history of breast cancer, but no additional samples were available for genotyping the relatives. The ExAC population frequency for the c.4025_4026 deletion in Finland is 0.015%.
Discussion
In this current case–control study, we evaluated the breast and ovarian cancer risk for the FANCM c.5791C>T mutation as well as for c.4025_4026delCT and c.5293dupA variants among Finnish population. We further examined the risk associated with carrying either of the FANCM mutations c.5101C>T and c.5791C>T.
The study revealed the FANCM c.5791C>T mutation being more frequent among the studied breast cancer cases than in controls in the Finnish population. It was particularly enriched among the TNBC cases, showing a significant association in the combined analysis (OR 5.14, P = 0.005). This observation is consistent with previous studies on FANCM and breast cancer risk, as the c.5101C>T mutation has also been found to associate with TNBC [12, 14]. Consistent results were seen also in the other subgroups studied (Table 2).
We further combined the results of the FANCM c.5101C>T risk analysis [12] with the current results of c.5791C>T to study the risk associated with carrying either of these C-terminal FANCM mutations. The risk was significantly increased in all subgroups of patients, especially among triple-negative cases (OR 3.08, 95% CI 1.77–5.35, P = 0.00007).
Studying the c.5791C>T mutation in the ovarian cancer cases showed an elevated risk (OR 1.60), but the association was not statistically significant. Previous studies on FANCM mutations have shown similar results [12, 14]; however, a very recent study found significant association between FANCM mutations and high grade serous ovarian cancer [36].
Screening of the other two FANCM variants exposed only one additional c.4025_4026delCT carrier and no c.5293dupA carriers among 862 familial breast cancer patients, and these were not studied further. However, their identification may suggest a wider mutation spectrum of very rare mutations in the FANCM gene, potentially relating to subtype-specific breast cancer predisposition.
Of all breast cancers, about 10–20% are found to be hormonally triple-negative and these are usually aggressive with a poor prognosis, as this subtype does not respond to hormonal therapy. These tumors are often higher grade and larger size than the other tumor subtypes [37]. Germline mutations in BRCA1 are common in TNBC cases, but deleterious changes in other homologous recombination DNA repair pathway genes have also been observed to occur relatively frequently in these patients, including BRCA2, PALB2, BARD1, and RAD51C [38]. The FA pathway has also been connected to TNBC, as comparison of mRNA expression in different breast tumor types revealed several FA pathway genes (BRCA1, FANCD2, FANCF, and PALB2) being significantly less expressed in TNBC tumors compared to luminal A (ER or PR positive) tumors [39]. This further supports the current finding that also FANCM depletion is linked especially with TNBC, but deeper understanding of this connection warrants further investigations. However, as the developmental mechanisms of TNBC are not well known, the identification of additional risk factors for this breast cancer subtype is valuable for understanding its etiology and for the identification of potential therapeutic targets.
FANCM has a crucial function in the DNA damage response for ICLs. It acts as a helicase/translocase and binds adjacent to the crosslink, inducing the recruitment of the FA core complex to the site. The core complex monoubiquitinates the FANCI–FANCD2 complex which triggers the accumulation of multiple nucleases and initiates the actual DNA repair processes [2]. Depletion of FANCM has been reported to have effects on both the FA pathway efficiency and tumorigenesis: FANCM deficient mouse embryonic fibroblasts displayed increased chromosomal breakage, residual FANCD2 monoubiquitination, and increased spontaneous sister chromatid exchanges, and the FANCM knockout mice had reduced overall and tumor-free survival [40].
The functional effect of FANCM c.5791C>T has previously been studied by Peterlongo et al. [13]. Instead of being a conventional nonsense mutation, it was proposed to create a binding site for a splicing factor hnRNP A1, which causes exon 22 skipping in mRNA and introduces a premature stop codon, leading to loss of 132 amino acids from the C-terminus of the protein. Two domains with important roles in binding of DNA, ERCC4 and helix–hairpin–helix (HhH)2, are located in the C-terminus of FANCM [41], and loss of them likely disturbs FANCM activity. Supporting this hypothesis, when the FANCM c.5791C>T mutation was introduced to mouse embryonic fibroblasts, the mutant cells displayed decreased DNA repair activity and increased chromosomal breakage [13]. This is consistent with the effect of the majority of the known breast cancer-associated gene mutations [42], further supporting the impact of FANCM c.5791C>T on genomic instability and increased cancer risk.
Altogether, the c.5791C>T mutation has to date been connected to familial breast cancer [13], and also to TNBC [14]. The current results on FANCM c.5791C>T, together with those of c.5101C>T, support a role for FANCM as a moderate-risk breast cancer susceptibility gene and further emphasize its connection with the triple-negative breast tumors.
Conclusions
The current results provide further support for the previously suggested association between FANCM mutations and triple-negative breast cancer. Further studies with larger datasets are needed for precise risk estimations. Also, other rare variants may occur in the FANCM gene and warrant further investigations in other populations.
Abbreviations
- FA:
-
Fanconi anemia
- ICL:
-
Interstrand crosslink
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- TNBC:
-
Triple-negative breast cancer
- ER:
-
Estrogen receptor
- PR:
-
Progesterone receptor
- HNPCC:
-
Hereditary non-polyposis colorectal cancer
References
Kee Y, D’Andrea AD (2012) Molecular pathogenesis and clinical management of Fanconi anemia. J Clin Invest 122:3799–3806
Michl J, Zimmer J, Tarsounas M (2016) Interplay between Fanconi anemia and homologous recombination pathways in genome integrity. EMBO J 35:909–923
Lim ET, Würtz P, Havulinna AS, Palta P, Tukiainen T, Rehnström K, Esko T, Mägi R, Inouye M, Lappalainen T, Chan Y, Salem RM, Lek M, Flannick J, Sim A, Manning A, Ladenvall C, Bumpstead S, Hämäläinen E, Aalto K, Maksimow M, Salmi M, Blankenberg S, Ardissino D, Shah S, Horne B, McPherson R, Hovingh GK, Reilly MP, Watkins H, Goel A, Farrall M, Girelli D, Reiner AP, Stitziel NO, Kathiresan S, Gabriel S, Barrett JC, Lehtimäki T, Laakso M, Groop L, Kaprio J, Perola M, McCarthy MI, Boehnke M, Altshuler DM, Lindgren CM, Hirschhorn JN, Metspalu A, Freimer NB, Zeller T, Jalkanen S, Koskinen S, Raitakari O, Durbin R, MacArthur DG, Salomaa V, Ripatti S, Daly MJ, Palotie A (2014) Sequencing Initiative Suomi (SISu) Project Distribution and medical impact of loss-of-function variants in the Finnish founder population. PLoS Genet 10:e1004494
Singh TR, Bakker ST, Agarwal S, Jansen M, Grassman E, Godthelp BC, Ali AM, Du CH, Rooimans MA, Fan Q, Wahengbam K, Steltenpool J, Andreassen PR, Williams DA, Joenje H, de Winter JP, Meetei AR (2009) Impaired FANCD2 monoubiquitination and hypersensitivity to camptothecin uniquely characterize Fanconi anemia complementation group M. Blood 114:174–180
Walsh T, King MC (2007) Ten genes for inherited breast cancer. Cancer Cell 11:103–105
Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T, Jayatilake H, McGuffog L, Hanks S, Evans DG, Eccles D (2007) Breast Cancer Susceptibility Collaboration (UK), Easton DF, Stratton MR. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 39:165–167
Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, Tomiak E, Neuhausen SL, Teo ZL, Khan S, Aittomäki K, Moilanen JS, Turnbull C, Seal S, Mannermaa A, Kallioniemi A, Lindeman GJ, Buys SS, Andrulis IL, Radice P, Tondini C, Manoukian S, Toland AE, Miron P, Weitzel JN, Domchek SM, Poppe B, Claes KB, Yannoukakos D, Concannon P, Bernstein JL, James PA, Easton DF, Goldgar DE, Hopper JL, Rahman N, Peterlongo P, Nevanlinna H, King MC, Couch FJ, Southey MC, Winqvist R, Foulkes WD, Tischkowitz M (2014) Breast-cancer risk in families with mutations in PALB2. N Engl J Med 371:497–506
Norquist BM, Harrell MI, Brady MF, Walsh T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Yi Q, Burger RA, Chan JK, Davidson SA, Mannel RS, DiSilvestro PA, Lankes HA, Ramirez NC, King MC, Swisher EM, Birrer MJ (2016) Inherited mutations in women with ovarian carcinoma. JAMA Oncol 2:482–490
Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, Niederacher D, Freund M, Lichtner P, Hartmann L, Schaal H, Ramser J, Honisch E, Kubisch C, Wichmann HE, Kast K, Deissler H, Engel C, Müller-Myhsok B, Neveling K, Kiechle M, Mathew CG, Schindler D, Schmutzler RK, Hanenberg H (2010) Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 42:410–414
Pelttari LM, Heikkinen T, Thompson D, Kallioniemi A, Schleutker J, Holli K, Blomqvist C, Aittomäki K, Bützow R, Nevanlinna H (2011) RAD51C is a susceptibility gene for ovarian cancer. Hum Mol Genet 15:3278–3288
Sawyer SL, Tian L, Kahkonen M, Schwartzentruber J, Kircher M, Majewski J, Dyment DA, Innes AM, Boycott KM, Moreau LA, Moilanen JS, Greenberg RA (2015) Biallelic mutations in BRCA1 cause a new Fanconi anemia subtype. Cancer Discov 5:135–142
Kiiski JI, Pelttari LM, Khan S, Freysteinsdottir ES, Reynisdottir I, Hart SN, Shimelis H, Vilske S, Kallioniemi A, Schleutker J, Leminen A, Bützow R, Blomqvist C, Barkardottir RB, Couch FJ, Aittomäki K, Nevanlinna H (2014) Exome sequencing identifies FANCM as a susceptibility gene for triple-negative breast cancer. Proc Natl Acad Sci USA 111:15172–15177
Peterlongo P, Catucci I, Colombo M, Caleca L, Mucaki E, Bogliolo M, Marin M, Damiola F, Bernard L, Pensotti V, Volorio S, Dall’Olio V, Meindl A, Bartram C, Sutter C, Surowy H, Sornin V, Dondon MG, Eon-Marchais S, Stoppa-Lyonnet D, Andrieu N, Sinilnikova OM, Mitchell G, James PA, Thompson E, Marchetti M, Verzeroli C, Tartari C, Capone GL, Putignano AL, Genuardi M, Medici V, Marchi I, Federico M, Tognazzo S, Matricardi L, Agata S, Dolcetti R, Della Puppa L, Cini G, Gismondi V, Viassolo V, Perfumo C, Mencarelli MA, Baldassarri M, Peissel B, Roversi G, Silvestri V, Rizzolo P, Spina F, Vivanet C, Tibiletti MG, Caligo MA, Gambino G, Tommasi S, Pilato B, Tondini C, Corna C, Bonanni B, Barile M, Osorio A, Benitez J, Balestrino L, Ottini L, Manoukian S, Pierotti MA, Renieri A, Varesco L, Couch FJ, Wang X, Devilee P, Hilbers FS, van Asperen CJ, Viel A, Montagna M, Cortesi L, Diez O, Balmana J, Hauke J, Schmutzler RK, Papi L, Pujana MA, Lazaro C, Falanga A, Offit K, Vijai J, Campbell I, Burwinkel B, Kvist A, Ehrencrona H, Mazoyer S, Pizzamiglio S, Verderio P, Surralles J, Rogan PK, Radice P (2015) FANCM c.5791C>T nonsense mutation (rs144567652) induces exon skipping, affects DNA repair activity and is a familial breast cancer risk factor. Hum Mol Genet 24:5345–5355
Neidhardt G, Hauke J, Ramser J, Groß E, Gehrig A, Müller CR, Kahlert AK, Hackmann K, Honisch E, Niederacher D, Heilmann-Heimbach S, Franke A, Lieb W, Thiele H, Altmüller J, Nürnberg P, Klaschik K, Ernst C, Ditsch N, Jessen F, Ramirez A, Wappenschmidt B, Engel C, Rhiem K, Meindl A, Schmutzler RK, Hahnen E (2016) Association between loss-of-function mutations within the FANCM gene and early-onset familial breast cancer. JAMA Oncol. doi:10.1001/jamaoncol.2016.5592
Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, O’Donnell-Luria AH, Ware JS, Hill AJ, Cummings BB, Tukiainen T, Birnbaum DP, Kosmicki JA, Duncan LE, Estrada K, Zhao F, Zou J, Pierce-Hoffman E, Berghout J, Cooper DN, Deflaux N, DePristo M, Do R, Flannick J, Fromer M, Gauthier L, Goldstein J, Gupta N, Howrigan D, Kiezun A, Kurki MI, Moonshine AL, Natarajan P, Orozco L, Peloso GM, Poplin R, Rivas MA, Ruano-Rubio V, Rose SA, Ruderfer DM, Shakir K, Stenson PD, Stevens C, Thomas BP, Tiao G, Tusie-Luna MT, Weisburd B, Won HH, Yu D, Altshuler DM, Ardissino D, Boehnke M, Danesh J, Donnelly S, Elosua R, Florez JC, Gabriel SB, Getz G, Glatt SJ, Hultman CM, Kathiresan S, Laakso M, McCarroll S, McCarthy MI, McGovern D, McPherson R, Neale BM, Palotie A, Purcell SM, Saleheen D, Scharf JM, Sklar P, Sullivan PF, Tuomilehto J, Tsuang MT, Watkins HC, Wilson JG, Daly MJ, MacArthur DG (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 17:285–291
Gracia-Aznarez FJ, Fernandez V, Pita G, Peterlongo P, Dominguez O, de la Hoya M, Duran M, Osorio A, Moreno L, Gonzalez-Neira A, Rosa-Rosa JM, Sinilnikova O, Mazoyer S, Hopper J, Lazaro C, Southey M, Odefrey F, Manoukian S, Catucci I, Caldes T, Lynch HT, Hilbers FS, van Asperen CJ, Vasen HF, Goldgar D, Radice P, Devilee P, Benitez J (2013) Whole exome sequencing suggests much of non-BRCA1/BRCA2 familial breast cancer is due to moderate and low penetrance susceptibility alleles. PLoS ONE 8:e55681
Smith CG, Naven M, Harris R, Colley J, West H, Li N, Liu Y, Adams R, Maughan TS, Nichols L, Kaplan R, Wagner MJ, McLeod HL, Cheadle JP (2013) Exome resequencing identifies potential tumor-suppressor genes that predispose to colorectal cancer. Hum Mutat 34:1026–1034
Huang M, Kennedy R, Ali AM, Moreau LA, Meetei AR, D’Andrea AD, Chen CC (2011) Human MutS and FANCM complexes function as redundant DNA damage sensors in the Fanconi anemia pathway. DNA Repair (Amst) 10:1203–1212
Broderick P, Dobbins SE, Chubb D, Kinnersley B, Dunlop MG, Tomlinson I, Houlston RS (2016) Validation of recently proposed colorectal cancer susceptibility gene variants in an analysis of families and patients-a systematic review. Gastroenterology 152:75–77
Syrjäkoski K, Vahteristo P, Eerola H, Tamminen A, Kivinummi K, Sarantaus L, Holli K, Blomqvist C, Kallioniemi OP, Kainu T, Nevanlinna H (2000) Population-based study of BRCA1 and BRCA2 mutations in 1035 unselected Finnish breast cancer patients. J Natl Cancer Inst 92:1529–1531
Kilpivaara O, Bartkova J, Eerola H, Syrjäkoski K, Vahteristo P, Lukas J, Blomqvist C, Holli K, Heikkilä P, Sauter G, Kallioniemi OP, Bartek J, Nevanlinna H (2005) Correlation of CHEK2 protein expression and c.1100delC mutation status with tumor characteristics among unselected breast cancer patients. Int J Cancer 113:575–580
Fagerholm R, Hofstetter B, Tommiska J, Aaltonen K, Vrtel R, Syrjäkoski K, Kallioniemi A, Kilpivaara O, Mannermaa A, Kosma VM, Uusitupa M, Eskelinen M, Kataja V, Aittomäki K, von Smitten K, Heikkilä P, Lukas J, Holli K, Bartkova J, Blomqvist C, Bartek J, Nevanlinna H (2008) NAD(P)H:quinone oxidoreductase 1 NQO1*2 genotype (P187S) is a strong prognostic and predictive factor in breast cancer. Nat Genet 40:844–853
Eerola H, Blomqvist C, Pukkala E, Pyrhönen S, Nevanlinna H (2000) Familial breast cancer in southern Finland: how prevalent are breast cancer families and can we trust the family history reported by patients? Eur J Cancer 36:1143–1148
Vahteristo P, Bartkova J, Eerola H, Syrjäkoski K, Ojala S, Kilpivaara O, Tamminen A, Kononen J, Aittomäki K, Heikkilä P, Holli K, Blomqvist C, Bartek J, Kallioniemi OP, Nevanlinna H (2002) A CHEK2 genetic variant contributing to a substantial fraction of familial breast cancer. Am J Hum Genet 71:432–438
Vehmanen P, Friedman LS, Eerola H, McClure M, Ward B, Sarantaus L, Kainu T, Syrjäkoski K, Pyrhönen S, Kallioniemi OP, Muhonen T, Luce M, Frank TS, Nevanlinna H (1997) Low proportion of BRCA1 and BRCA2 mutations in Finnish breast cancer families: evidence for additional susceptibility genes. Hum Mol Genet 6:2309–2315
Vahteristo P, Eerola H, Tamminen A, Blomqvist C, Nevanlinna H (2001) A probability model for predicting BRCA1 and BRCA2 mutations in breast and breast-ovarian cancer families. Br J Cancer 84:704–708
Eerola H, Heikkilä P, Tamminen A, Aittomäki K, Blomqvist C, Nevanlinna H (2005) Histopathological features of breast tumours in BRCA1, BRCA2 and mutation-negative breast cancer families. Breast Cancer Res 7:R93–R100
Sarantaus L, Vahteristo P, Bloom E, Tamminen A, Unkila-Kallio L, Bützow R, Nevanlinna H (2001) BRCA1 and BRCA2 mutations among 233 unselected Finnish ovarian carcinoma patients. Eur J Hum Genet 9:424–430
Hartikainen JM, Tuhkanen H, Kataja V, Dunning AM, Antoniou A, Smith P, Arffman A, Pirskanen M, Easton DF, Eskelinen M, Uusitupa M, Kosma VM, Mannermaa A (2005) An autosome-wide scan for linkage disequilibrium-based association in sporadic breast cancer cases in eastern Finland: three candidate regions found. Cancer Epidemiol Biomark Prev 14:75–80
Kauppinen JM, Kosma VM, Soini Y, Sironen R, Nissinen M, Nykopp TK, Karja V, Eskelinen M, Kataja V, Mannermaa A (2010) ST14 gene variant and decreased matriptase protein expression predict poor breast cancer survival. Cancer Epidemiol Biomark Prev 19:2133–2142
Mantere T, Haanpää M, Hanenberg H, Schleutker J, Kallioniemi A, Kähkönen M, Parto K, Avela K, Aittomäki K, von Koskull H, Hartikainen JM, Kosma VM, Laasanen SL, Mannermaa A, Pylkäs K, Winqvist R (2015) Finnish Fanconi anemia mutations and hereditary predisposition to breast and prostate cancer. Clin Genet 88:68–73
Pylkäs K, Vuorela M, Otsukka M, Kallioniemi A, Jukkola-Vuorinen A, Winqvist R (2012) Rare copy number variants observed in hereditary breast cancer cases disrupt genes in estrogen signaling and TP53 tumor suppression network. PLoS Genet 8:e1002734
Brunet J (2010) Hereditary breast cancer and genetic counseling in young women. Breast Cancer Res Treat 123(SUPPL. 1):7–9
Tervasmäki A, Winqvist R, Pylkäs K (2014) Recurrent CYP2C19 deletion allele is associated with triple-negative breast cancer. BMC Cancer 14:902
Mantere T, Winqvist R, Kauppila S, Grip M, Jukkola-Vuorinen A, Tervasmäki A, Rapakko K, Pylkäs K (2016) Targeted next-generation sequencing identifies a recurrent mutation in MCPH1 associating with hereditary breast cancer susceptibility. PLoS Genet 12:e1005816
Dicks E, Song H, Ramus SJ, Van Oudenhove E, Tyrer JP, Intermaggio MP, Kar S, Harrington P, Bowtell DD, Cicek MS, Cunningham JM, Fridley BL, Alsop J, Jimenez-Linan M, Piskorz A, Goranova T, Kent E, Siddiqui N, Paul J, Crawford R, Poblete S, Lele S, Sucheston-Campbell L, Moysich KB, Sieh W, McGuire V, Lester J, Odunsi K, Whittemore AS, Bogdanova N, Dürst M, Hillemanns P, Karlan BY, Gentry-Maharaj A, Menon U, Tischkowitz M, Levine D, Brenton JD, Dörk T, Goode EL, Gayther SA, Pharoah PDP (2017) Germline whole exome sequencing and large-scale replication identified FANCM as a likely high grade serous ovarian cancer susceptibility gene. Oncotarget. doi:10.18632/oncotarget.15871
Albergaria A, Ricardo S, Milanezi F, Carneiro V, Amendoeira I, Vieira D, Cameselle-Teijeiro J, Schmitt F (2011) Nottingham prognostic index in triple-negative breast cancer: a reliable prognostic tool? BMC Cancer 11:299
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, Olson JE, Godwin AK, Pankratz VS, Olswold C, Slettedahl S, Hallberg E, Guidugli L, Davila JI, Beckmann MW, Janni W, Rack B, Ekici AB, Slamon DJ, Konstantopoulou I, Fostira F, Vratimos A, Fountzilas G, Pelttari LM, Tapper WJ, Durcan L, Cross SS, Pilarski R, Shapiro CL, Klemp J, Yao S, Garber J, Cox A, Brauch H, Ambrosone C, Nevanlinna H, Yannoukakos D, Slager SL, Vachon CM, Eccles DM, Fasching PA (2015) Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J Clin Oncol 1:304–311
Ribeiro E, Ganzinelli M, Andreis D, Bertoni R, Giardini R, Fox SB, Broggini M, Bottini A, Zanoni V, Bazzola L, Foroni C, Generali D, Damia G (2013) Triple negative breast cancers have a reduced expression of DNA repair genes. PLoS ONE 25:e66243
Bakker ST, van de Vrugt HJ, Rooimans MA, Oostra AB, Steltenpool J, Delzenne-Goette E, van der Wal A, van der Valk M, Joenje H, te Riele H, de Winter JP (2009) Fancm-deficient mice reveal unique features of Fanconi anemia complementation group M. Hum Mol Genet 18:3484–3495
Whitby MC (2010) The FANCM family of DNA helicases/translocases. DNA Repair 3:224–236
Davis JD, Lin SY (2011) DNA damage and breast cancer. World J Clin Oncol 10:329–338
Acknowledgements
The authors wish to thank all the volunteered patients participating in this study. Helsinki Breast Cancer Study thanks research nurses Irja Erkkilä and Virpi Palola for their help with collecting patient data and samples. For the cancer diagnostic data, The Finnish Cancer Registry is gratefully acknowledged. HEBCS wish also to thank the staff at the Technology Centre, Institute for Molecular Medicine Finland (FIMM), for SNV marker genotyping. Oulu breast cancer study thanks Annika Väntänen and Leena Keskitalo for technical assistance.
Funding
Helsinki breast cancer study has been supported by the Helsinki University Central Hospital Research Fund, the Academy of Finland (266528), the Sigrid Juselius Foundation, the Cancer Society of Finland, the Finnish Cultural Foundation for LMP, and the Biomedicum Helsinki Foundation for JK. Oulu breast cancer study has been supported by the Academy of Finland (250083) for KP, and by the Academy of Finland (122715 and Center of Excellence 284605), the Cancer Society of Finland, the Sigrid Juselius Foundation, the University of Oulu, the University of Oulu Support Foundation, and the special Governmental EVO funds for Oulu University Hospital-based research activities for RW. The Kuopio breast cancer study was supported by the special Government Funding of Kuopio University Hospital Grants, The Cancer Society of Finland, and the strategic fund of the University of Eastern Finland.
Author information
Authors and Affiliations
Contributions
JIK, LMP, and HN designed the study. JIK and AT performed the molecular genetic studies. JIK analyzed and pooled the data. JIK and AT carried out the statistical analyses and drafted the manuscript with HN. LMP, SK, TM, KP, AM, MT, AK, ÅB, V-MK, AK, JS, RB, CB, KA, and RW contributed samples, data and patient information. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kiiski, J.I., Tervasmäki, A., Pelttari, L.M. et al. FANCM mutation c.5791C>T is a risk factor for triple-negative breast cancer in the Finnish population. Breast Cancer Res Treat 166, 217–226 (2017). https://doi.org/10.1007/s10549-017-4388-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4388-0