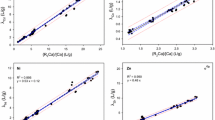
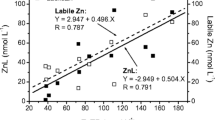
Abstract
Combining in situ diffusion and column ion-exchange equilibration, we measured free metal ion concentrations (Cd, Cu and Zn) in water samples collected from the epilimnion of 14 lakes in the Rouyn-Noranda area (600 km north-west of Montreal, QC, Canada). Lakes were selected to represent a wide range of physico-chemical characteristics (hardness, pH, dissolved organic matter—DOM, degree of metal contamination), to determine the influence of these parameters on metal speciation. Total dissolved metal concentrations, as determined within the diffusion cells, varied over one to two orders of magnitude: [Cd] 0.19–2.9 nM; [Cu] 36–190 nM; [Zn] 7–2,800 nM. The proportion of total dissolved metal present as free Cd2+ and Zn2+ was relatively constant for the 14 selected lakes, despite the wide pH (4.5–8) and DOM (3–23 mg C/L) ranges, probably reflecting the inverse relationship observed between pH and DOM; this proportion did, however, vary with DOM and pH for Cu. Our experimental free metal ion concentrations were compared with those calculated with the thermodynamic models WHAM (Windermere Humic Aqueous Model VI) and ECOSAT 4.7 (incorporating the NICA-Donnan model). Measured and calculated values were in reasonable agreement for both Cd and Zn although measured values were generally slightly higher, i.e. less than one order of magnitude. For several lakes, measured free Cu concentrations were, however, much higher than the calculated values, suggesting that these models overestimate Cu complexation. The gap between measured and calculated free metal ion concentration becomes more important as the total metal concentration decreases and as pH increases.




Similar content being viewed by others
Notes
Although we subtracted the mean free metal ion concentration measured using analytical blanks (Cd: 0.030 ± 0.017 nM; Cu: 0.14 ± 0.03 nM; Zn: 6.6 ± 3.1 nM; N = 6), the high variability in Zn contamination, presumably due to sample handling despite all precautions taken, strongly affected some of the low Zn samples.
References
Beneš P, Steinnes E (1974) In situ dialysis for the determination of the state of trace elements in natural waters. Water Res 8:947–953
Bio-Rad (1988) Guide to ion exchange. Hercules, California
Brown GK, Cabaniss SE, MacCarthy P, Leenheer JA (1999) Cu(II) binding by a pH-fractionated fulvic acid. Anal Chim Acta 402:183–193
Buffle J (1988) Complexation reactions in aquatic systems. An analytical approach. Wiley, Chichester
Campbell PGC (1995) Interactions between trace metals and aquatic organisms: a critique of the free-ion activity model. In: Tessier A, Turner DR (eds) Metal speciation and bioavailability in aquatic systems. Wiley, New York, pp 45–102
Cantwell FF, Nielsen JS, Hrudrey SE (1982) Free nickel ion concentration in sewage by an ion exchange column-equilibration method. Anal Chem 54:1498–1503
Cao J, Xue HB, Sigg L (2006) Effects of pH and Ca competition on complexation of cadmium by fulvic acids and by natural organic ligands from a river and a lake. Aquat Geochem 12:375–387
Chen Y, Senesi N, Schnitzer M (1977) Information provided on humic substances by E4/E6 ratios. Soil Sci Soc Am J 41:352–358
Cheng T, Allen HE (2006) Comparison of zinc complexation properties of dissolved natural organic matter from different surface waters. J Environ Manag 80:222–229
Cheng T, De Schamphelaere K, Lofts S, Janssen C, Allen HE (2005) Measurement and computation of zinc binding to natural dissolved organic matter in European surface waters. Anal Chim Acta 542:230–239
Christensen JB, Christensen TH (1999) Complexation of Cd, Ni, and Zn by DOC in polluted groundwater: A comparison of approaches using resin exchange, aquifer material sorption, and computer speciation models (WHAM and MINTEQA2). Environ Sci Technol 33:3857–3863
Christensen JB, Botma JJ, Christensen TH (1999) Complexation of Cu and Pb by DOC in polluted groundwater: A comparison of experimental data and predictions by computer speciation models (WHAM and MINTEQA2). Water Res 33:3231–3238
Couillard Y, Campbell PGC, Tessier A (1993) Response of metallothionein concentrations in a freshwater bivalve (Anodonta grandis) along an environmental cadmium gradient. Limnol Oceanogr 38:299–313
Croteau M-N, Hare L, Tessier A (2002) Increases in food web cadmium following reductions in atmospheric inputs to some lakes. Environ Sci Technol 36:3079–3082
Diamond RM, Whitney DC (1966) Resin selectivity in aqueous solutions: ion exchange. In: Marinsky JA (ed) Ion exchange. Dekker, New York, pp 277–351
Doig LE, Liber K (2007) Nickel speciation in the presence of different sources and fractions of dissolved organic matter. Ecotoxicol Environ Saf 66:169–177
Fortin C, Campbell PGC (1998) An ion-exchange technique for free-metal ion measurements (Cd2+, Zn2+): applications to complex aqueous media. Int J Environ Anal Chem 72:173–194
Fortin C, Caron F (2000) Complexing capacity of low-level radioactive waste leachates for Co-60 and Cd-109 using an ion-exchange technique. Anal Chim Acta 410:107–117
Ge Y, MacDonald D, Sauvé S, Hendershot W (2005a) Modeling of Cd and Pb speciation in soil solutions by WinhumicV and NICA-Donnan model. Environ Modell Softw 20:353–359
Ge Y, Sauvé S, Hendershot WH (2005b) Equilibrium speciation of cadmium, copper, and lead in soil solutions. Commun Soil Sci Plant Anal 36:1537–1556
Giguère A, Campbell PGC, Hare L, McDonald DG, Rasmussen JB (2004) Influence of lake chemistry and fish age on cadmium, copper, and zinc concentrations in various organs of indigenous yellow perch (Perca flavescens). Can J Fish Aquat Sci 61:1702–1716
Gopalapillai Y, Fasfous II, Murimboh JD, Yapici T, Chakraborty P, Chakrabarti CL (2008) Determination of free nickel ion concentrations using the ion exchange technique: application to aqueous mining and municipal effluents. Aquat Geochem 14:99–116
Guthrie JW, Hassan NM, Salam MSA, Fasfous II, Murimboh CA, Murimboh J, Chakrabarti CL, Grégoire DC (2005) Complexation of Ni, Cu, Zn, and Cd by DOC in some metal-impacted freshwater lakes: a comparison of approaches using electrochemical determination of free-metal-ion and labile complexes and a computer speciation model, WHAM V and VI. Anal Chim Acta 528:205–218
Hamilton-Taylor J, Postill AS, Tipping E, Harper MP (2002) Laboratory measurements and modeling of metal-humic interactions under estuarine conditions. Geochim Cosmochim Acta 66:403–415
Hering JG, Morel FMM (1988) Humic acid complexation of calcium and copper. Environ Sci Technol 22:1234–1237
Holm PE, Christensen TH, Tjell JC, McGrath SP (1995) Speciation of cadmium and zinc with application to soil solutions. J Environ Qual 24:183–190
Horowitz AJ, Lum KR, Garbarino JR, Hall GEM, Lemieux C, Demas CR (1996) Problems associated with using filtration to define dissolved trace element concentrations in natural water samples. Environ Sci Technol 30:954–963
Kalis EJJ, Weng LP, Dousma F, Temminghoff EJM, Van Riemsdijk WH (2006) Measuring free metal ion concentrations in situ in natural waters using the Donnan Membrane Technique. Environ Sci Technol 40:955–961
Keizer MG, Van Riemsdijk WH (1999) A computer program for the equilibrium calculation of speciation and transport in soil-water systems (ECOSAT 4.7). Wageningen University, The Netherlands
Kinniburgh DG, Milne CJ, Benedetti MF, Pinheiro JP, Filius J, Koopal LK, van Riemsdik WH (1996) Metal ion binding by humic acid: application of the NICA-Donnan model. Environ Sci Technol 30:1687–1698
Li Y-H, Gregory S (1974) Diffusion of ions in sea water and in deep-sea sediments. Geochim Cosmochim Acta 38:703–714
Mantoura RFC, Dickson A, Riley JP (1978) The complexation of metals with humic materials in natural waters. Estuarine Costal Mar Sci 6:387–408
Martell AE, Smith RM, Motekaitis RJ (2004) NIST critical stability constants of metal complexes database. US Department of Commerce, Gaithersburg, MD, USA
Meylan S, Odzak N, Behra R, Sigg L (2004) Speciation of copper and zinc in natural freshwater: comparison of voltammetric measurements, diffusive gradients in thin films (DGT) and chemical equilibrium models. Anal Chim Acta 510:91–100
Morel FMM, Hering JG (eds) (1993) Principles and applications of aquatic chemistry. Wiley, New York
Néron R, Auclair JC, Fortin C (2006) Rate of Cd2+ release from dissolved fulvic acid and natural dissolved organic carbon as a function of UVB dose. Environ Chem 3:433–438
Nolan AL, McLaughlin MJ, Mason SD (2003) Chemical speciation of Zn, Cd, Cu, and Pb in pore waters of agricultural and contaminated soils using Donnan dialysis. Environ Sci Technol 37:90–98
Oste LA, Temminghoff EJM, Lexmond TM, van Riemsdik WH (2002) Measuring and modeling zinc and cadmium binding by humic acid. Anal Chem 74:856–862
Perceval O, Pinel-Alloul B, Methot G, Couillard Y, Giguère A, Campbell PGC, Hare L (2002) Cadmium accumulation and metallothionein synthesis in freshwater bivalves (Pyganodon grandis): relative influence of the metal exposure gradient versus limnological variability. Environ Pollut 118:5–17
Perceval O, Couillard Y, Pinel-Alloul B, Giguère A, Campbell PGC (2004) Metal-induced stress in bivalves living along a gradient of Cd contamination: relating sub-cellular metal distribution to population-level responses. Aquat Toxicol 69:327–345
Perceval O, Couillard Y, Pinel-Alloul B, Bonneris E, Campbell PG (2006) Long-term trends in accumulated metals (Cd, Cu and Zn) and metallothionein in bivalves from lakes within a smelter-impacted region. Sci Total Environ 369:403–418
Perdue EM, Ritchie JD (2003) Dissolved organic matter in freshwaters. In: Drever JI (ed) Surface and ground water, weathering, and soils. Elsevier, Amsterdam, pp 273–318
Rhee IH, Dzombak DA (1999) Binary and ternary cation exchange on strong acid cation exchange resin involving Na, Mg, and Zn in single and binary backgrounds of chloride, perchlorate, and sulfate. Langmuir 15:6875–6883
Rozan TF, Benoit G (1999) Geochemical factors controlling free Cu ion concentrations in river water. Geochim Cosmochim Acta 63:3311–3319
Rozan TF, Benoit G, Marsh H, Chin Y-P (1999) Intercomparison of DPASV and ISE for the measurement of Cu complexation characteristics of NOM in freshwater. Environ Sci Technol 33:1766–1770
Sherwood GD, Rasmussen JB, Rowan DJ, Brodeur J, Hontela A (2000) Bioenergetic costs of heavy metal exposure in Yellow Perch (Perca flavescens): in situ estimates with a radiotracer (Cs-137) technique. Can J Fish Aquat Sci 57:441–450
Sigg L, Black F, Buffle J, Cao J, Cleven R, Davison W, Galceran J, Gunkel P, Kalis E, Kistler D, Martin M, Noel S, Nur Y, Odzak N, Puy J, Van Riemsdijk W, Temminghoff E, Tercier-Waeber ML, Toepperwien S, Town RM, Unsworth E, Warnken KW, Weng LP, Xue HB, Zhang H (2006) Comparison of analytical techniques for dynamic trace metal speciation in natural freshwaters. Environ Sci Technol 40:1934–1941
Sweileh JA, Lucyk D, Kratochvil B, Cantwell FF (1987) Specificity of the ion exchange—atomic absorption method for free copper(II) determination in natural waters. Anal Chem 59:586–592
Tipping E (1998) Humic ion-binding model VI: an improved description of the interactions of protons and metal ions with humic substances. Aquat Geochem 4:3–48
Tipping E (2002) Cation binding by humic substances. Cambridge University Press, Cambridge
Tipping E, Rey-Castro C, Bryan SE, Hamilton-Taylor J (2002) Al(III) and Fe(III) binding by humic substances in freshwaters, and implications for trace metal speciation. Geochim Cosmochim Acta 66:3211–3224
Unsworth ER, Zhang H, Davison W (2005) Use of diffusive gradients in thin films to measure cadmium speciation in solutions with synthetic and natural ligands: comparison with model predictions. Environ Sci Technol 39:624–630
Unsworth ER, Warnken KW, Zhang H, Davison W, Black F, Buffle J, Cao J, Cleven R, Galceran J, Gunkel P, Kalis E, Kistler D, VanLeeuwen HP, Martin M, Noël S, Nur Y, Odzak N, Puy J, Van Riemsdijk W, Sigg L, Temminghoff E, Tercier-Waeber ML, Toepperwien S, Town RM, Weng LP, Xue HB (2006) Model predictions of metal speciation in freshwaters compared to measurements by in situ techniques. Environ Sci Technol 40:1942–1949
Vigneault B, Campbell PGC (2005) Uptake of cadmium by freshwater green algae: effects of pH and aquatic humic substances. J Phycol 41:55–61
Wang DC, Couillard Y, Campbell PGC, Jolicoeur P (1999) Changes in subcellular metal partitioning in the gills of freshwater bivalves (Pyganodon grandis) living along an environmental cadmium gradient. Can J Fish Aquat Sci 56:774–784
Worms IAM, Wilkinson KJ (2008) Determination of Ni2+ using an equilibrium ion exchange technique: Important chemical factors and applicability to environmental samples. Anal Chim Acta 616:95–102
Xue HB, Sigg L (1999) Comparison of the complexation of Cu and Cd by humic or fulvic acids and by ligands observed in lake waters. Aquat Geochem 5:313–335
Xue HB, Nhat PH, Sigg L (2005) Effects of soil composition on Zn speciation in drainage waters from agricultural soils. Aquat Geochem 11:303–318
Zhang H (2004) In situ speciation of Ni and Zn in freshwaters: Comparison between DGT measurements and speciation models. Environ Sci Technol 38:1421–1427
Zhang H, Davison W (1995) Performance characteristics of diffusion gradients in thin films for the in situ measurement of trace metals in aqueous solution. Anal Chem 67:3391–3400
Acknowledgments
The authors acknowledge the technical assistance provided by M.G. Bordeleau, F. Beauchamp, R. Néron, M. Guillot and M. Arsenault in the laboratory and R. Rodrigue, J. Orvoine, O. Perceval, I. Louis, A. Giguère, A. Dumoulin, and A. van den Abeele in the field. Comments provided by A. Tessier and K.K. Mueller on earlier versions of the MS were greatly appreciated. Financial support was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC strategic research grant STP 0192937) and by the Fonds québécois de la recherche sur la nature et les technologies (FQRNT team grant). P.G.C. Campbell is supported by the Canada Research Chair Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortin, C., Couillard, Y., Vigneault, B. et al. Determination of Free Cd, Cu and Zn Concentrations in Lake Waters by In Situ Diffusion Followed by Column Equilibration Ion-exchange. Aquat Geochem 16, 151–172 (2010). https://doi.org/10.1007/s10498-009-9074-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10498-009-9074-3