Abstract
Objective
The aim of this study was to analyze the capacity of Artemin promoting the motility and invasiveness of MIA PaCa-2 pancreatic cancer (PAC) cells.
Methods
The PAC cell line MIA PaCa-2 was cultured in vitro and studied using Transwell chamber analysis. The motility and invasiveness ability affected by different concentrations of Artemin and its receptor GFRα3 were determined. Expression level of matrix metalloproteinase-2 (MMP-2), epithelial cadherin (E-cadherin) were quantitative analysis using RT-PCR and Western blot in MIA PaCa-2 cells stimulated with Artemin and receptor GFRα3.
Results
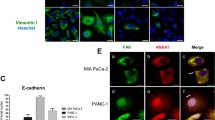
MIA PaCa-2 PAC cell motility and invasiveness was significantly increased with Artemin and its receptor GFRα3 increasing concentrations than control (P < 0.01). 150 ng/mL was the best of both the role of concentration. MMP-2 was increased significantly (t = 6.35, t = 7.32), while E-cadherin was significantly lower (t = 4.27, t = 5.61), after affected by the 150 ng/mL Artemin and GFRα3, respectively. The difference was statistically significant compared with the control group (P < 0.01).
Conclusion
Artemin and its receptor GFRα3 can promote PAC cell motility and invasiveness ability and contribute to the aggressive behavior. The mechanism may be related to increased expression of MMP-2 molecule and E-cadherin down-regulation expression.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin, 2009, 59: 225–249.
Lomberk G. Pain management. Pancreatology, 2008, 8: 542–543.
Airaksinen MS, Holm L, Hätinen T. Brain Behav Evol, 2006, 68: 181–190.
Rückert F, Görgens H, Richter I, et al. RET-protooncogene variants in patients with sporadic neoplasms of the digestive tract and the central nervous system. Int J Colorectal Dis, 2011, 26: 835–840.
Meng LX, LI Q, Xue YJ, et al. Effects of nerve growth factor on invasive ability of human pancreatic cancer cell line MIA PaCa-2. Chin J Hepatobiliary Surg (Chinese), 2008, 14: 796–800.
Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic-cancer cells bearing c-ret protooncogene with reference to glial-cell-line-derived neurotrophic factor (GDNF). Int J Cancer, 1999, 81: 67–73.
Baloh RH, Tansey MG, Lampe PA, et al. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron, 1998, 21: 1291–1302.
Enomoto H, Crawford PA, Gorodinsky A, et al. RET signaling is essential for migration, axonal growth and axon guidance of developing sympathetic neurons. Development, 2001, 128: 3963–3974.
Honma Y, Araki T, Gianino S, et al. Artemin is a vascular-derived neurotropic factor for developing sympathetic neurons. Neuron, 2002, 35: 267–282.
Andres R, Forgie A, Wyatt S, et al. Multiple effects of artemin on sympathetic neurone generation, survival and growth. Development, 2001, 128: 3685–3695.
Baloh RH, Tansey MG, Johnson EM, et al. Functional mapping of receptor specificity domains of glial cell line-derived neurotrophic factor (GDNF) family ligands and production of GFRalpha1 RET-specific agonists. J Biol Chem, 2000, 275: 3412–3420.
Ceyhan GO, Giese NA, Erkan M, et al. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg, 2006, 244: 274–281.
Orozco OE, Walus L, Sah DW, et al. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci, 2001, 13: 2177–2182.
Takahashi M. The GDNF/RET signaling pathway and human diseases. Cytokine Growth Factor Rev, 2001, 12: 361–373.
Terada T, Okada Y, Nakanuma Y. Expression of matrix proteinases during human intrahepatic bile duct development. A possible role in biliary cell migration. Am J Pathol, 1995, 147: 1207–1213.
Jo Chae K, Rha SY, Oh BK, et al. Expression of matrix metalloproteinase-2 and -9 and tissue inhibitor of metalloproteinase-1 and -2 in intraductal and nonintraductal growth type of cholangiocarcinoma. Am J Gastroenterol, 2004, 99: 68–75.
McLaughlin RB, Montone KT, Wall SJ, et al. Nerve cell adhesion molecule expression in squamous cell carcinoma of the head and neck: a predictor of propensity toward perineural spread. Laryngoscope, 1999, 109: 821–826.
von Burstin J, Eser S, Paul MC, et al. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology, 2009, 137: 361–371.
Torer N, Kayaselcuk F, Nursal TZ, et al. Adhesion molecules as prognostic markers in pancreatic adenocarcinoma. J Surg Oncol, 2007, 96: 419–423.
Winter JM, Ting AH, Vilardell F, et al. Absence of E-cadherin expression distinguishes noncohesive from cohesive pancreatic cancer. Clin Cancer Res, 2008, 14: 412–418.
Kao WT, Lin CY, Lee LT, et al. Investigation of MMP-2 and -9 in a highly invasive A431 tumor cell sub-line selected from a Boyden chamber assay. Anticancer Res, 2008, 28: 2109–2120.
Veit C, Genze F, Menke A, et al. Activation of phosphatidylinositol 3-kinase and extracellular signal-regulated kinase is required for glial cell line-derived neurotrophic factor-induced migration and invasion of pancreatic carcinoma cells. Cancer Res, 2004, 64: 5291–5300.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by grants from the National Science Foundation of Shandong Province (No. ZR2010HL053) and the Surface Project of Jining Medical University
Rights and permissions
About this article
Cite this article
Meng, L., Chi, Y., Wang, X. et al. The neurotrophic factor Artemin promotes the motility and invasiveness of MIA PaCa-2 pancreatic cancer cells. Chin. -Ger. J. Clin. Oncol. 11, 219–223 (2012). https://doi.org/10.1007/s10330-011-0955-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10330-011-0955-8