Abstract
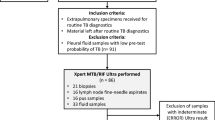
Tuberculosis (TB) is a worldwide public health concern, including in high-resource countries with a low prevalence of TB. Xpert MTB/RIF assay was developed to improve TB and rifampicin (RIF) resistance detection, but sensitivity remains poor on smear-negative sputum. Xpert MTB/RIF Ultra assay was designed to enhance the sensitivity of TB detection in clinical samples. Herein, we evaluated retrospectively the performance of this test on smear-negative respiratory samples. Respiratory specimens with smear-negative and a Mycobacterium tuberculosis (MTB) complex-positive culture were retrospectively selected from those taken from patients during routine care, and analysed in the Mycobacteria Laboratory of the Lyon University hospital, France. Specimens were stored at − 20 °C before testing by Xpert MTB/RIF Ultra. For each sample, growth delay and date of anti-TB treatment initiation were recorded. Forty-six samples—29 sputum, 8 bronchial aspirates, 6 broncho-alveolar lavages, and 3 gastric aspirates—were selected. Among samples collected before treatment initiation (n = 33), sensitivity was 81.8% (95% CI [64.5; 93.0]) and there was a significant correlation between the quantitative measurements (Ct) of Xpert MTB/RIF Ultra assay and the time to growth detection in culture. Among samples collected after treatment initiation (n = 12), sensitivity was 100%, without correlation with time to growth detection due to presence of afterglow DNA in samples. In high-resource settings, the Xpert MTB/RIF Ultra test represents a useful tool for pulmonary TB diagnosis, notably for the paucibacillary forms. Moreover, quantitative measurement of Xpert MTB/RIF Ultra could help to predict time to MTB culture positivity and be used as a quality indicator of MTB culture process.

Similar content being viewed by others
References
Global tuberculosis Report (2017). World Health Organization
Forbes BA, Hall GS, Miller MB, Novak SM, Rowlinson M-C, Salfinger M (2018) et al. Practice guidelines for clinical microbiology laboratories: mycobacteria. Clin Microbiol Rev 31. https://doi.org/10.1128/CMR.00038-17
Centers for Disease Control and Prevention (CDC) (2009) Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis. MMWR Morb Mortal Wkly Rep 58:7–10
WHO Meeting Report of a Technical Expert. Consultation: Non-inferirity analysis of Xpert MTB/RIF Ultra compared to Xpert MTB/RIF. World Health Organization;
Sohn H, Aero AD, Menzies D, Behr M, Schwartzman K, Alvarez GG et al (2014) Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis 58:970–976. https://doi.org/10.1093/cid/ciu022
Steingart KR, Schiller I, Horne DJ, Pai M, Boehme CC, Dendukuri N (2014) Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 21:CD009593. https://doi.org/10.1002/14651858.CD009593.pub3
Nicol MP, Workman L, Prins M, Bateman L, Ghebrekristos Y, Mbhele S, et al. (2018) Accuracy of Xpert MTB/RIF Ultra for the diagnosis of pulmonary tuberculosis in children. Pediatr Infect Dis J ;37(10):e261-e263. https://doi.org/10.1097/INF.0000000000001960
Rufai SB, Singh A, Singh J, Kumar P, Sankar MM, Singh S et al (2017) Diagnostic usefulness of Xpert MTB/RIF assay for detection of tuberculous meningitis using cerebrospinal fluid. J Inf Secur 75:125–131. https://doi.org/10.1016/j.jinf.2017.04.010
Chakravorty S, Simmons AM, Rowneki M, Parmar H, Cao Y, Ryan J et al (2017) The new Xpert MTB/RIF Ultra: improving detection of Mycobacterium tuberculosis and resistance to rifampin in an assay suitable for point-of-care testing. mBio 29:8. https://doi.org/10.1128/mBio.00812-17
Final WHO Report on Expert Technical Consultation FINAL23032017.docx - WHO-HTM-TB-2017.04-eng.pdf [Internet]. [cited 2017 27]; http://apps.who.int/iris/bitstream/10665/254792/1/WHO-HTM-TB-2017.04-eng.pdf?ua=1. Accessed 27 Nov 2017
Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A et al (2017) Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis 18(1):68–75. https://doi.org/10.1016/S1473-3099(17)30474-7
Dorman SE, Schumacher SG, Alland D, Nabeta P, Armstrong DT, King B et al (2018) Xpert MTB/RIF Ultra for detection of Mycobacterium tuberculosis and rifampicin resistance: a prospective multicentre diagnostic accuracy study. Lancet Infect Dis 18:76–84. https://doi.org/10.1016/S1473-3099(17)30691-6
Berhanu RH, David A, da Silva P, Shearer K, Sanne I, Stevens W, et al. (2018) Performance of Xpert MTB/RIF, Xpert Ultra, and Abbott real time MTB for the diagnosis of pulmonary tuberculosis in a high HIV burden setting. J Clin Microbiol 56:e00560–18. https://doi.org/10.1128/JCM.00560-18
Perez-Risco D, Rodriguez-Temporal D, Valledor-Sanchez I, Alcaide F (2018) Evaluation of the Xpert MTB/RIF Ultra assay for direct detection of Mycobacterium tuberculosis complex in smear-negative extrapulmonary samples. J Clin Microbiol 56:e00659–18. https://doi.org/10.1128/JCM.00659-18
Bisognin F, Lombardi G, Lombardo D, Re MC, Dal Monte P (2018) Improvement of Mycobacterium tuberculosis detection by Xpert MTB/RIF Ultra: a head-to-head comparison on Xpert-negative samples. PLoS One 13:e0201934. https://doi.org/10.1371/journal.pone.0201934
Ratnam S, Stead FA, Howes M (1987) Simplified acetylcysteine-alkali digestion-decontamination procedure for isolation of mycobacteria from clinical specimens. J Clin Microbiol 25:1428–1432
Smithwick RW, Bigbie MR, Ferguson RB, Karlix MA, Wallis CK (1995) Phenolic acridine orange fluorescent stain for mycobacteria. J Clin Microbiol 33:2763–2764
Macondo EA, Ba F, Gaye-Diallo A, Touré-Kane NC, Kaïré O, Gueye-Ndiaye A et al (2000) Rapid susceptibility testing of Mycobacterium tuberculosis by the mycobacteria growth indicator tube (MGIT AST SIRE). Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis 6:363–367
Lee H-S, Kee S-J, Shin J-H, Kwon Y-S, Chun S, Lee JH, et al. (2018) Xpert MTB/RIF assay as a substitute for smear microscopy in an intermediate burden setting. Am J Respir Crit Care Med. 25; https://doi.org/10.1164/rccm.201804-0654OC
Arend SM, van Soolingen D (2018) Performance of Xpert MTB/RIF Ultra: a matter of dead or alive. Lancet Infect Dis 18:8–10. https://doi.org/10.1016/S1473-3099(17)30695-3
Kendall EA, Schumacher SG, Denkinger CM, Dowdy DW (2017) Estimated clinical impact of the Xpert MTB/RIF Ultra cartridge for diagnosis of pulmonary tuberculosis: a modeling study. PLoS Med 14:e1002472. https://doi.org/10.1371/journal.pmed.1002472
Acknowledgements
We thank the ECCMID (European Congress of Clinical Microbiology and Infectious Diseases) 2018 Programme Committee to have accepted this work for ECCMID 2018 congress (ePoster session), and Philip Robinson (DRCI, Hospices Civils de Lyon) for help in manuscript preparation. We thank Cepheid for having provided the Xpert MTB/RIF Ultra cartridges used for this study.
Author information
Authors and Affiliations
Contributions
EH, CG, IF, JPR, GL, and OD designed the experiments. EH, AM, LC, and CB performed the experiments. EH, GL, and OD wrote the manuscript.
Corresponding author
Ethics declarations
This study was in accordance with the ethics committee of the Lyon University hospital, France (declared sample collection: DC-2011-1306). In accordance with French legislation, written informed patient consent was not required to compare technical performance of assays with clinical specimens collected following clinical recommendation.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hodille, E., Maisson, A., Charlet, L. et al. Evaluation of Xpert MTB/RIF Ultra performance for pulmonary tuberculosis diagnosis on smear-negative respiratory samples in a French centre. Eur J Clin Microbiol Infect Dis 38, 601–605 (2019). https://doi.org/10.1007/s10096-018-03463-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-018-03463-1