Abstract
Introduction
Patients with Parkinson’s disease (PD) present a variety of non-motor symptoms. However, it remains unclear whether dopamine depletion is related to non-motor symptoms, and which non-motor symptoms are significantly dependent on dopaminergic deficit.
Methods
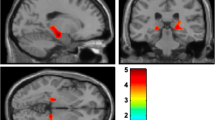
Forty-one patients with PD who underwent positron emission tomography imaging of dopamine transporters (DATs) were recruited for this study. The striatum was divided into 12 subregions, and DAT activity, as striatal dopaminergic concentration, was calculated in each subregion. In addition to measuring motor symptoms using the Unified Parkinson’s Disease Rating Scale-part III (UPDRS-III), various non-motor symptoms were assessed using the Montreal cognitive assessment, frontal assessment battery, Beck depression inventory (BDI), Beck anxiety inventory, PD sleep scale (PDSS), PD fatigue scale, and non-motor symptoms scale (NMSS) for PD.
Results
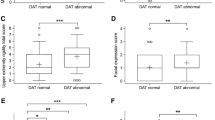
For simple linear regression analyses, dopaminergic depletion in all striatal subregions was negatively correlated with the UPDRS-III score. The most relevant non-motor symptom assessment related to dopaminergic loss in the 12 subregions was NMSS, followed by BDI and PDSS. However, following multiple linear regression analyses, dopaminergic depletion in the 12 striatal subregions was not related with any of the non-motor symptoms. Conversely, dopaminergic deficit in the right anterior and posterior putamen was associated with the UPDRS-III score.
Conclusions
Striatal dopaminergic depletion was not significantly correlated with any of the various non-motor symptoms in PD. Our findings suggest that non-dopaminergic systems are significantly implicated in the pathogenesis of non-motor symptoms in patients with PD.
Similar content being viewed by others
References
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9(1):13–24
Kaasinen V, Vahlberg T (2017) Striatal dopamine in Parkinson disease: a meta-analysis of imaging studies. Ann Neurol 82(6):873–882
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67(6):715–725
Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, Dhawan V, Feigin A, Fahn S, Guttman M, Gwinn-Hardy K, McFarland H, Innis R, Katz RG, Kieburtz K, Kish SJ, Lange N, Langston JW, Marek K, Morin L, Moy C, Murphy D, Oertel WH, Oliver G, Palesch Y, Powers W, Seibyl J, Sethi KD, Shults CW, Sheehy P, Stoessl AJ, Holloway R (2005) The role of radiotracer imaging in Parkinson disease. Neurology 64(2):208–215
Seibyl JP, Marek KL, Quinlan D, Sheff K, Zoghbi S, Zea-Ponce Y, Baldwin RM, Fussell B, Smith EO, Charney DS, van Dyck C et al (1995) Decreased single-photon emission computed tomographic [123I]beta-CIT striatal uptake correlates with symptom severity in Parkinson’s disease. Ann Neurol 38(4):589–598
Reichmann H, Brandt MD, Klingelhoefer L (2016) The nonmotor features of Parkinson’s disease: pathophysiology and management advances. Curr Opin Neurol 29:467–473
Lee HM, Koh SB (2015) Many faces of Parkinson’s disease: non-motor symptoms of Parkinson’s disease. J Mov Disord 8(2):92–97
Pont-Sunyer C, Hotter A, Gaig C, Seppi K, Compta Y, Katzenschlager R, Mas N, Hofeneder D, Brucke T, Bayes A, Wenzel K, Infante J, Zach H, Pirker W, Posada IJ, Alvarez R, Ispierto L, De Fabregues O, Callen A, Palasi A, Aguilar M, Marti MJ, Valldeoriola F, Salamero M, Poewe W, Tolosa E (2015) The onset of nonmotor symptoms in Parkinson’s disease (the ONSET PD study). Mov Disord 30(2):229–237
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Oh M, Kim JS, Kim JY, Shin KH, Park SH, Kim HO, Moon DH, Oh SJ, Chung SJ, Lee CS (2012) Subregional patterns of preferential striatal dopamine transporter loss differ in Parkinson disease, progressive supranuclear palsy, and multiple-system atrophy. J Nucl Med 53(3):399–406
Lee JY, Dong Woo L, Cho SJ, Na DL, Hong Jin J, Kim SK, You Ra L, Youn JH, Kwon M, Lee JH, Maeng Je C (2008) Brief screening for mild cognitive impairment in elderly outpatient clinic: validation of the Korean version of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol 21(2):104–110
Kim TH, Huh Y, Choe JY, Jeong JW, Park JH, Lee SB, Lee JJ, Jhoo JH, Lee DY, Woo JI, Kim KW (2010) Korean version of frontal assessment battery: psychometric properties and normative data. Dement Geriatr Cogn Disord 29(4):363–370
Jo SA, Park MH, Jo I, Ryu SH, Han C (2007) Usefulness of Beck Depression Inventory (BDI) in the Korean elderly population. Int J Geriatr Psychiatry 22(3):218–223
Lee K, Kim D, Cho Y (2018) Exploratory factor analysis of the Beck anxiety inventory and the Beck depression inventory-II in a psychiatric outpatient population. J Korean Med Sci 33(16):e128
Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, Pezzela FR, Forbes A, Hogl B, Trenkwalder C (2002) The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry 73:629–635
Brown RG, Dittner A, Findley L, Wessely SC (2005) The Parkinson fatigue scale. Parkinsonism Relat Disord 11:49–55
Koh SB, Kim JW, Ma HI, Ahn TB, Cho JW, Lee PH, Chung SJ, Kim JS, Kwon DY, Baik JS (2012) Validation of the korean-version of the nonmotor symptoms scale for Parkinson’s disease. J Clin Neurol 8:276–283
Kim HW, Kim JS, Oh M, Oh JS, Lee SJ, Oh SJ, Chung SJ, Lee CS (2016) Different loss of dopamine transporter according to subtype of multiple system atrophy. Eur J Nucl Med Mol Imaging 43:517–525
Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, Van Heertum R, Laruelle M (2001) Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 21:1034–1057
Razek AA, Elmongy A, Hazem M, Zakareyia S, Gabr W (2011) Idiopathic Parkinson disease effect of levodopa on apparent diffusion coefficient value of the brain. Acad Radiol 18(1):70–73
Abdel Razek AA, Abd El-Gaber N, Abdalla A, Fathy A, Azab A, Rahman AA (2009) Apparent diffusion coefficient vale of the brain in patients with Gaucher’s disease type II and type III. Neuroradiology 51(11):773–779
El-mewafy Z, Abdel Razek A, El-Eshmawy M, Abo El-Eneen N, EL-Biaomy A (2018) MR spectroscopy of the frontal region in patients with metabolic syndrome: correlation with anthropometric measurement. Polish J Radiol 83:e215–e219
Chung SJ, Yoo HS, Oh JS, Kim JS, Ye BS, Sohn YH, Lee PH (2018) Effect of striatal dopamine depletion on cognition in de novo Parkinson’s disease. Parkinsonism Relat Disord 51:43–48
Pellecchia MT, Picillo M, Santangelo G, Longo K, Moccia M, Erro R, Amboni M, Vitale C, Vicidomini C, Salvatore M, Barone P, Pappata S (2015) Cognitive performances and DAT imaging in early Parkinson’s disease with mild cognitive impairment: a preliminary study. Acta Neurol Scand 131(5):275–281
Kubler D, Schroll H, Buchert R, Kuhn AA (2017) Cognitive performance correlates with the degree of dopaminergic degeneration in the associative part of the striatum in non-demented Parkinson’s patients. J Neural Transm (Vienna) 124(9):1073–1081
Siepel FJ, Bronnick KS, Booij J, Ravina BM, Lebedev AV, Pereira JB, Gruner R, Aarsland D (2014) Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord 29(14):1802–1808
Rinne JO, Portin R, Ruottinen H, Nurmi E, Bergman J, Haaparanta M, Solin O (2000) Cognitive impairment and the brain dopaminergic system in Parkinson disease: [18F]fluorodopa positron emission tomographic study. Arch Neurol 57(4):470–475
Weintraub D, Newberg AB, Cary MS, Siderowf AD, Moberg PJ, Kleiner-Fisman G, Duda JE, Stern MB, Mozley D, Katz IR (2005) Striatal dopamine transporter imaging correlates with anxiety and depression symptoms in Parkinson’s disease. J Nucl Med 46(2):227–232
Erro R, Pappata S, Amboni M, Vicidomini C, Longo K, Santangelo G, Picillo M, Vitale C, Moccia M, Giordano F, Brunetti A, Pellecchia MT, Salvatore M, Barone P (2012) Anxiety is associated with striatal dopamine transporter availability in newly diagnosed untreated Parkinson’s disease patients. Parkinsonism Relat Disord 18(9):1034–1038
Ceravolo R, Frosini D, Poletti M, Kiferle L, Pagni C, Mazzucchi S, Volterrani D, Bonuccelli U (2013) Mild affective symptoms in de novo Parkinson’s disease patients: relationship with dopaminergic dysfunction. Eur J Neurol 20(3):480–485
Vriend C, Raijmakers P, Veltman DJ, van Dijk KD, van der Werf YD, Foncke EM, Smit JH, Berendse HW, van den Heuvel OA (2014) Depressive symptoms in Parkinson’s disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J Neurol Neurosurg Psychiatry 85(2):159–164
Felicio AC, Moriyama TS, Godeiro-Junior C, Shih MC, Hoexter MQ, Borges V, Silva SM, Amaro-Junior E, Andrade LA, Ferraz HB, Bressan RA (2010) Higher dopamine transporter density in Parkinson’s disease patients with depression. Psychopharmacology 211(1):27–31
Rektorova I, Srovnalova H, Kubikova R, Prasek J (2008) Striatal dopamine transporter imaging correlates with depressive symptoms and tower of London task performance in Parkinson’s disease. Mov Disord 23(11):1580–1587
Santangelo G, Vitale C, Picillo M, Cuoco S, Moccia M, Pezzella D, Erro R, Longo K, Vicidomini C, Pellecchia MT, Amboni M, Brunetti A, Salvatore M, Barone P, Pappata S (2015) Apathy and striatal dopamine transporter levels in de-novo, untreated Parkinson’s disease patients. Parkinsonism Relat Disord 21(5):489–493
Chung SJ, Lee JJ, Ham JH, Lee PH, Sohn YH (2016) Apathy and striatal dopamine defects in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord 23:62–65
Yousaf T, Pagano G, Niccolini F, Politis M (2018) Excessive daytime sleepiness may be associated with caudate denervation in Parkinson disease. J Neurol Sci 387:220–227
Chung SJ, Lee JJ, Ham JH, Ye BS, Lee PH, Sohn YH (2016) Striatal dopamine depletion patterns and early non-motor burden in Parkinsons disease. PLoS One 11(8):e0161316
Maillet A, Krack P, Lhommee E, Metereau E, Klinger H, Favre E, Le Bars D, Schmitt E, Bichon A, Pelissier P, Fraix V, Castrioto A, Sgambato-Faure V, Broussolle E, Tremblay L, Thobois S (2016) The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 139(Pt 9):2486–2502
Boileau I, Warsh JJ, Guttman M, Saint-Cyr JA, McCluskey T, Rusjan P, Houle S, Wilson AA, Meyer JH, Kish SJ (2008) Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: a preliminary PET study with [11C]DASB. Mov Disord 23(12):1776–1780
Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ (2010) Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133(11):3434–3443
Funding
This work was supported by the Soonchunhyang University Research Fund and the National Research Foundation (NRF) of Republic of Korea (No. NRF-2018R1C1B5045312).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments.
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Park, S.B., Kwon, KY., Lee, JY. et al. Lack of association between dopamine transporter loss and non-motor symptoms in patients with Parkinson’s disease: a detailed PET analysis of 12 striatal subregions. Neurol Sci 40, 311–317 (2019). https://doi.org/10.1007/s10072-018-3632-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-018-3632-7