Abstract
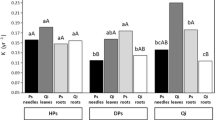
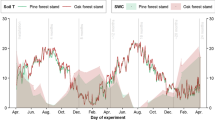
Tree species can affect the decomposition process through the quality of their leaf fall and through the species-specific conditions that they generate in their environment. We compared the relative importance of these effects in a 2-year experiment. Litterbags containing leaf litter of the winter-deciduous Quercus canariensis, the evergreen Q. suber and mixed litter were incubated beneath distinct plant covers. We measured litter carbon loss, 9 macro- and micronutrients and 18 soil chemical, physical and biological parameters of the incubation environment. Tree species affected decay dynamics through their litter quality and, to a lesser extent, through the induced environmental conditions. The deciduous litter showed a faster initial decomposition but left a larger fraction of slow decomposable biomass compared with the perennial litter; in contrast the deciduous environment impeded early decomposition while promoting further carbon loss in the latter decay stages. The interaction of these effects led to a negative litter–environment interaction contradicting the home-field advantage hypothesis. Leaf litter N, Ca and Mn as well as soil N, P and soil moisture were the best predictors for decomposition rates. Litter N and Ca exerted counteractive effects in early versus late decay stages; Mn was the best predictor for the decomposition limit value, that is, the fraction of slowly decomposable biomass at the later stage of decomposition; P and soil moisture showed a constant and positive relation with carbon loss. The deciduous oak litter had a higher initial nutrient content and released its nutrients faster and in a higher proportion than the perennial oak litter, significantly increasing soil fertility beneath its canopy. Our findings provide further insights into the factors that control the early and late stages of the decomposition process and reveal potential mechanisms underlying tree species influence on litter decay rate, carbon accumulation and nutrient cycling.





Similar content being viewed by others
References
Anonymous. 2005. PORN/PRUG/PDS Parque Natural Los Alcornocales. Junta de Andalucía, Consejería de Medio Ambiente, Sevilla, España.
Aponte C, García LV, Marañón T, Gardes M. 2010a. Indirect host effect on ectomycorrhizal fungi: leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks. Soil Biol Biochem 42:788–96.
Aponte C, Marañón T, García LV. 2010b. Microbial C, N and P in soils of Mediterranean oak forests: influence of season, canopy cover and soil depth. Biogeochemistry 101:77–92.
Aponte C, García LV, Pérez-Ramos IM, Gutiérrez E, Marañón T. 2011. Oak trees and soil interactions in Mediterranean forests: a positive feedback model. J Veg Sci 22:856–67.
Attiwill PM. 1968. The loss of elements from decomposing litter. Ecology 49:142–5.
Augusto L, Ranger J, Binkley D, Rothe A. 2002. Impact of several common tree species of European temperate forests on soil fertility. Ann For Sci 59:233–53.
Austin AT, Vivanco L. 2006. Plant litter decomposition in a semi-arid ecosystem controlled by photodegradation. Nature 442:555–8.
Ayres E, Steltzer H, Berg S, Wall DH. 2009. Soil biota accelerate decomposition in high-elevation forests by specializing in the breakdown of litter produced by the plant species above them. J Ecol 97:901–12.
Bennett LT, Kasel S, Tibbits J. 2009. Woodland trees modulate soil resources and conserve fungal diversity in fragmented landscapes. Soil Biol Biochem 41:2162–9.
Berg B. 1986. Nutrient release from litter and humus in coniferous forest soils: a mini review. Scand J For Res 1:359–69.
Berg B. 2000. Litter decomposition and organic matter turnover in northern forest soils. For Ecol Manage 133:13–22.
Berg B, Ekbohm G. 1991. Litter mass-loss rates and decomposition patterns in some needle and leaf litter types. Long-term decomposition in a Scots pine forest VII. Can J Bot 69:1449–56.
Berg B, McClaugherty C. 2008. Plant litter. Heidelberg: Springer.
Berg B, Ekbohm G, Johansson M-B, McClaugherty C, Rutigliano F, De Virzo Santo A. 1996. Maximum decomposition limits of forest litter types: a synthesis. Can J Bot 74:659–72.
Berg B, De Santo AV, Rutigliano FA, Fierro A, Ekbohm G. 2003. Limit values for plant litter decomposing in two contrasting soils: influence of litter elemental composition. Acta Oecologica 24:295–302.
Berg B, Steffen K, McClaugherty C. 2007. Litter decomposition rate is dependent on litter Mn concentrations. Biogeochemistry 82:29–39.
Berg B, Davey M, De Marco A, Emmett B, Faituri M, Hobbie S, Johansson MB, Liu C, McClaugherty C, Norell L, Rutigliano F, Vesterdal L, De Virzo Santo A. 2010. Factors influencing limit values for pine needle litter decomposition: a synthesis for boreal and temperate pine forest systems. Biogeochemistry 100:57–73.
Blair J. 1988. Nutrient release from decomposing foliar litter of three tree species with special reference to calcium, magnesium and potassium dynamics. Plant Soil 110:49–55.
Brookes PC, Landman A, Pruden G, Jenkinson DS. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–42.
Chadwick DR, Ineson P, Woods C, Piearce TG. 1998. Decomposition of Pinus sylvestris litter in litter bags: influence of underlying native litter layer. Soil Biol Biochem 30:47–55.
Cornelissen JHC, Quested HM, van Logtestijn RSP, Pérez-Harguindeguy N, Gwynn-Jones D, Díaz S, Callaghan TV, Press MC, Aerts R. 2006. Foliar pH as a new plant trait: can it explain variation in foliar chemistry and carbon cycling processes among subarctic plant species and types? Oecologia 147:315–26.
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Bodegom Pv, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–71.
Davey MP, Berg B, Emmett BA, Rowland P. 2007. Decomposition of oak leaf litter is related to initial litter Mn concentrations. Can J Bot 85:16–24.
Enríquez S, Duarte CM, Sand-Jensen K. 1993. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94:457–71.
Eriksson K-E, Blanchette RA, Ander P. 1990. Microbial and enzymatic degradation of wood and wood components. Berlin: Springer.
Finzi AC, Canham CD, Van Breemen N. 1998a. Canopy tree–soil interactions within temperate forests: species effects on pH and cations. Ecol Appl 8:447–54.
Finzi AC, Van Breemen N, Canham CD. 1998b. Canopy tree–soil interactions within temperate forests: species effects on soil carbon and nitrogen. Ecol Appl 8:440–6.
Gallardo A, Merino J. 1993. Leaf decomposition in two Mediterranean ecosystems of southwest Spain: influence of substrate quality. Ecology 74:152–61.
García LV. 2003. Controlling the false discovery rate in ecological research. Trends Ecol Evol 18:553–4.
Gartner TB, Cardon ZG. 2004. Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–46.
Güsewell S, Gessner MO. 2009. N: P ratios influence litter decomposition and colonization by fungi and bacteria in microcosms. Funct Ecol 23:211–19.
Hansen R. 1999. Red oak litter promotes a microarthropod functional group that accelerates its decomposition. Plant Soil 209:37–45.
Hatakka A. 2005. Biodegradation of lignin. Biopolymers online. Weinheim: Wiley.
Hättenschwiler S, Gasser P. 2005. Soil animals alter plant litter diversity effects on decomposition. Proc Natl Acad Sci USA 102:1519–24.
Hobbie SE. 1992. Effects of plant species on nutrient cycling. Trends Ecol Evol 7:336–9.
Hobbie SE. 1996. Temperature and plant species control over litter decomposition in Alaskan tundra. Ecol Monogr 66:503–22.
Hobbie SE. 2008. Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–44.
Hobbie SE, Vitousek PM. 2000. Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–77.
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P. 2006. Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87:2288–97.
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P. 2012. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr (in press).
Karberg NJ, Scott NA, Giardina CP. 2008. Methods for estimating litter decomposition. In: Hoover CM, Ed. Field measurements for forest carbon monitoring. New York: Springer. p 103–11.
Laskowski R, Niklińska M, Maryański M. 1995. The dynamics of chemical elements in forest litter. Ecology 76:1393–406.
Maheswaran J, Attiwill PM. 1987. Loss of organic matter, elements, and organic fractions in decomposing Eucalyptus microcarpa leaf litter. Can J Bot 65:2601–6.
McClaugherty C, Pastor J, Aber J, Melillo J. 1985. Forest litter decomposition in relation to soil nitrogen dynamics and litter quality. Ecology 66:266–75.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Mitchell RJ, Campbell CD, Chapman SJ, Osler GHR, Vanbergen AJ, Ross LC, Cameron CM, Cole L. 2007. The cascading effects of birch on heather moorland: a test for the top-down control of an ecosystem engineer. J Ecol 93:540–54.
Negrete-Yankelevich S, Fragoso C, Newton A, Russell G, Heal O. 2008. Species-specific characteristics of trees can determine the litter macroinvertebrate community and decomposition process below their canopies. Plant Soil 307:83–97.
Ojeda F, Marañón T, Arroyo J. 2000. Plant diversity patterns in the Aljibe Mountains (S. Spain): a comprehensive account. Biodivers Conserv 9:1323–43.
Ostrofsky ML. 2007. A comment on the use of exponential decay models to test nonadditive processing hypotheses in multispecies mixtures of litter. J North Am Benthol Soc 26:23–7.
Perez J, Jeffries TW. 1992. Roles of manganese and organic acid chelators in regulating lignin degradation and biosynthesis of peroxidases by Phanerochaete chrysosporium. Appl Environ Microbiol 58:2402–9.
Pérez-Ramos IM, Zavala MA, Marañón T, Díaz-Villa MD, Valladares F. 2008. Dynamics of understorey herbaceous plant diversity following shrub clearing of cork oak forests: a five-year study. For Ecol Manage 255:3242–53.
Prescott CE. 1995. Does nitrogen availability control rates of litter decomposition in forests? Plant Soil 168–169:83–8.
Reich PB, Oleksyn J, Modrzynski J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG. 2005. Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–18.
Sariyildiz T, Anderson JM. 2003. Interactions between litter quality, decomposition and soil fertility: a laboratory study. Soil Biol Biochem 35:391–9.
Soil Survey Staff. 2010. Keys to soil taxonomy. 11th edn. Washington, DC: USDA-Natural Resources Conservation Service.
Sparks DL. 1996. Methods of soil analysis. Part 3. Madison: Chemical Methods Soil Science Society of America and American Society of Agronomy.
Staaf H, Berg B. 1982. Accumulation and release of plant nutrients in decomposing Scots pine needle litter. Long-term decomposition in a Scots pine forest II. Can J Bot 60:1561–8.
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA. 2009. Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–36.
Swift MJ, Heal OW, Anderson JM. 1979. Decomposition in terrestrial ecosystems. Los Angeles: University of California Press.
Vance ED, Brookes PC, Jenkinson DS. 1987. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–7.
Vesterdal L, Raulund-Rasmussen K. 1998. Forest floor chemistry under seven tree species along a soil fertility gradient. Can J For Res 28:1636–47.
Vivanco L, Austin AT. 2008. Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–36.
Wieder RK, Lang GE. 1982. A critique of the analytical methods used in examining decomposition data obtained from litter bags. Ecology 63:1636–42.
Acknowledgments
We thank the Consejería de Medio Ambiente (Andalusian Government) and Marco Antonio Tena, then Director of Los Alcornocales Natural Park, for the facilities and support to carry out our field work. We are grateful to Eduardo Gutiérrez, Susana Hito, Marga Santaella, and Daniel Caballos for field and lab assistance. We would also like to thank S. Hobbie, subject-matter editor, and two anonymous reviewers for their constructive comments on the original manuscript. This study was supported by a FPI-MEC Grant to C.A., by the Spanish projects DINAMED (CGL2005-5830-C03-01), INTERBOS (CGL2008-4503-C03-01), GESBOME (P06-RNM-1890), and Subprograma de Técnicos de Apoyo MICINN (PTA2009-1782-I) and European FEDER funds.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author contributions
CA, LVG, and TM conceived of the idea and wrote the article; CA conducted chemical analyses and analyzed the data.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Aponte, C., García, L.V. & Marañón, T. Tree Species Effect on Litter Decomposition and Nutrient Release in Mediterranean Oak Forests Changes Over Time. Ecosystems 15, 1204–1218 (2012). https://doi.org/10.1007/s10021-012-9577-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-012-9577-4