Abstract
Lactobacillus paracasei has been demonstrated to inhibit the growth of many pathogenic microbes such as Streptococcus mutans, in vitro. However, its clinical application remains unclear. Here, we examined whether a novel probiotic L. paracasei GMNL-33 may reduce the caries-associated salivary microbial counts in healthy adults. Seventy-eight subjects (aged 20 to 26) had completed this double-blinded, randomized, placebo-controlled study. A probiotic/test (n = 42) and a control group (n = 36) took a L. paracasei GMNL-33 and a placebo oral tablet three times per day for 2 weeks, respectively. Bacterial counts of salivary S. mutans, lactobacilli, and salivary buffer capacity were measured with chair-side kits at the beginning (T1), the completion (T2) of medication, and 2 weeks after medication (T3). The results did not show differences in the counts of S. mutans and lactobacilli between probiotic and control groups at T1, T2, and T3. Nevertheless, within the probiotic group, an interesting probiotic effect was noticed. Between T1 and T2, no inhibitory effect against S. mutans was observed. However, a significant count reduction in the salivary S. mutans was detected between T2 and T3 (p = 0.016). Thus, a 2-week period of medication via oral administration route may be needed for L. paracasei GMNL-33 to be effective in the probiotic action.
Similar content being viewed by others
Introduction
The definition of “probiotics” has been adopted by the International Scientific Association and the World Health Organization: “Live microorganisms, if administered in adequate amounts, confer a health benefit on the host” [1]. Recently, a lot of studies focused on the effect of the probiotic for oral health. Among previous human clinical studies, Lactobacillus rhamnosus GG, ATCC 53103 (LGG) and L. rhamnosus LC 705 [2], Lactobacillus reuteri [3–6], and Bifidobacterium [7, 8] could inhibit the oral cariogenic bacteria such as Streptococcus mutans. LGG and L. rhamnosus LC 705 could reduce the prevalence of yeast counts in elder persons [9]. Lactobacillus salivarius TI 2711 could reduce Porphyromonas gingivalis counts [10]. L. reuteri could reduce gingivitis and plaque scores [11]. Weissella confusa CMU [12] and Streptococcus salivarius K12 [13] could reduce halitosis in human study.
Lactobacillus paracasei isolated from healthy humans showed antibacterial and anticandidal activities against oral pathogens such as S. mutans, S. salivarius, Streptococcus sanguis, Staphylococcus aureus, Actinomyces viscosus, P. gingivalis, Candida albican, Candida tropicalis, and Candida grabata [14]. The strongest antimicrobial activity was attributed to L. paracasei, Lactobacillus plantarum, L. rhamnosus, and L. salivarius isolated from the persons who had chronic periodontitis or health periodontium [15]. Lactobacilli isolated from the caries-free instead of caries-prone subjects had a significantly superior capacity to suppress the growth of S. mutans; L. paracasei was one of the Lactobacillus species with the maximum interference activity against S. mutans in vitro [16].
According to these findings, a new product containing probiotics L. paracasei aimed to reduce S. mutans was designed. However, its ability for reduction of cariogenic pathogens in human is unclear. A randomized human study to evaluate the efficacy of this probiotic L. paracasei for dental health is necessary.
The purpose of this study was to evaluate the efficacy of the probiotic L. paracasei GMNL-33 for reducing caries-associated salivary microbial counts in healthy adults. S. mutans was determined to play a major role in the initiation of caries lesions. Certain Lactobacillus species were believed to have a part in the caries progression. The salivary buffer capacity value would also be detected as supplement indicator. The change of the counts of these two microbials and the salivary buffer capacity has been utilized in documented investigations for caries risk evaluation. Thus, the two indicators would be deemed adequate to assess the efficacy of the L. paracasei for caries prevention. The null hypothesis was that the probiotic L. paracasei GMNL-33 would not alter the bacterial levels and salivary buffer capacity compared with placebo controls.
Materials and methods
The study protocol was approved by the Institutional Reviewing Broad (IRB 96-0311B) and Chang Gung Medical Research Project (XMRPG 460021) at Human Investigation Committee of Chang Gung Memorial Hospital and Chang Gung University. Informed consent from each subject was obtained prior to the clinical trial.
Participants
Eighty healthy volunteer subjects, aged 20–40 years, were recruited for the study. Grouping by randomized, double-blind method, there were 42 and 38 samples for the probiotic and control group, respectively, at the beginning of study. The inclusion criteria were healthy adults without any prescribed medication. The exclusion criteria were persons with smoking, systemic disease, long-term use of antibiotics, or undergoing dental treatment including orthodontic, periodontal, endodontic, and prosthodontic treatment.
Study protocol and the intervention
The study was a randomized, double-blind, placebo-controlled study with two parallel groups. The oral tablets for the probiotic group and control group were randomized, numbered from first to ninth according to a computer-generated blocked, randomization list. For blinding purposes, the packaging of both oral tablets was identical. A total of 80 participants were randomized to receive one package of oral tablets. The probiotic oral tablets, commercial name as Dental-Lac, contained 11% xylitol and 4% exact of L. paracasei GMNL-33 bacteria 3 × 108 cells/tablet (1 g). The control tablets contained 11% xylitol without probiotic strain. All of these oral tablets were prepared by GenMont Biotech Incorporation, Taiwan. Subjects were instructed to take one oral tablet and dissolve it in the mouth slowly after meals three times a day for 2 weeks. Throughout the study, they were supposed to turn in a diary about how often they had taken the oral tablets.
The study consisted of two 2-week periods: a 2-week intervention period (T1–T2) and a 2-week posttreatment period (T2–T3). Bacterial counts of salivary S. mutans, lactobacilli, and salivary buffer capacity were evaluated with chair-side kits at baseline (T1), at the completion (T2) of medication and 2 weeks after medication (T3).
The subjects could use toothbrushes, fluoride toothpaste, and dental floss during the study period. The use of other fluoride products, similar probiotic products, and other medical oral rinse was forbidden. The participants were told to brush their teeth twice a day.
Clinical examination
The subjects received an oral examination before the study by two experienced dentists. The clinical examination was conducted by using the oral mirror and Community Periodontal Index probe under the natural light and according to the WHO criteria (World Health Organization, Oral Health Surveys Basic Method 4th ed.). The number of decayed/missing/filled teeth (DMFT) was recorded.
Saliva samples
Salivary microbials were collected three times at week 0, 2, and 4 around noon time (11:00 a.m.–1:00 p.m.). The subjects were told to refrain from eating and drinking for 2 h prior to the investigation.
Salivary counts of S. mutans were determined by using the Dentocult SM Strip mutans® slides (Orion Diagnostica, Finland). One of the examiners placed a bacitracin tablet in the selective culture broth about 15 min before examination. Subjects chewed on a paraffin pellet for 1 min and were instructed to swallow any excess saliva. They then pressed the rough surface of the strip against the saliva remaining on the tongue. The strip that immersed with saliva was placed in the selective culture broth. We incubated the vial in an upright position at 37°C for 48 h with the cap one quarter of a turn open. After incubation, the presence of S. mutans was evidenced by dark blue to light blue, raised colonies on the rough surface of the strip. The density of S. mutans was obtained by comparing the colony density on the test strip with the model chart: score = 0 for density less than or equal to 104 CFU/ml, score = 1 for density between 104 and 105 CFU/ml, score = 2 for density between 105 and 106 CFU/ml, and score = 3 for density greater than 106 CFU/ml.
Salivary counts of lactobacilli were determined by using the Dentocult LB Dip Slide® method (Orion Diagnostica, Finland). We let the subjects chew on a paraffin pellet for 2 min and collected the stimulated saliva. Some drops of stimulated saliva were pipetted on to both surfaces of the agar dip slides. We incubated the vial in an upright position at 37°C for 4 days. The results were presented as: score = 0 for density equal to 103 CFU/ml, score = 1 for density equal to 104 CFU/ml, score = 2 for density equal to 105 CFU/ml, and score = 3 for density equal to 106 CFU/ml.
There were three clinicians evaluating the test kits. The agreement of the final measurement values of S. mutans and lactobacilli with three clinicians was 0.79 to 0.88 and 0.69 to 0.85, respectively. The validity of these methods has found to be good [17].
Salivary buffering capacity was determined by using the Dentobuff® Strip method (Orion Diagnostica, Finland). A drop of stimulated saliva was pipetted onto the pad, and the result was read after 5 min. The color of this test pad was compared with a standard color chart after 5 min to estimate the final pH. The method grades stimulated saliva as low (pH ≤4), intermediate (pH 4.5–5.5), or high (pH ≥6) buffer capacity. The results were presented as: score = 0 for low pH, score = 1 for intermediate pH, and score = 2 for high pH.
Statistical analysis
Inter-group differences in salivary S. mutans counts, salivary lactobacilli counts, and buffer capacity were analyzed by Mann–Whitney U Test. Intra-group differences in three stages of salivary S. mutans counts, salivary lactobacilli counts, and salivary buffer capacity were analyzed by the Wilcoxon signed-rank test. Fisher’s exact test was applied to test the percentage of change in salivary S. mutans counts, salivary lactobacilli counts, and salivary buffer capacity. All the analyses were performed via Statistical Package for Social Sciences (SPSS version 12.1 Inc., Chicago, IL, USA) software. The level of statistical significance was set at p = 0.05.
Results
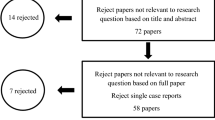
There were 80 adult volunteers involved the clinical trial and the number of investigated subjects were given in the flow chart (Fig. 1). Two participants of the control group dropped out during the study period. There were 42 subjects (21 female and 21 male, average age 21.0 ± 1.2 years old, DMFT 7.2 ± 5.2) in the probiotic group and 36 subjects (21 female and 17 males, average age 21.1 ± 2.1 years old, DMFT 5.4 ± 3.4) in the control group. The DMFT values between the two groups were analyzed by Mann–Whitney U test. There were no significant differences in the DMFT values between the two groups (p = 0.218).
The compliance of subjects was reported by themselves through a record chart for oral tablets intake frequency. The compliance results were presented as: “excellent” for eating all tablets, “good” for only missing 1∼5 tablets, “moderate” for missing 6∼10 tablets, and “poor” for missing more than 10 tablets. The excellent compliance was 41.33%, good was 40.00%, moderate was 12.00%, and poor was 6.67%.
The comparison results of S. mutans counts, lactobacilli counts, and buffer capacity were not significant between probiotic and control groups at T1, T2, and T3. The data was analyzed by Mann–Whitney U test. (Table 1)
Within the probiotic group, the S. mutans counts did not change during T1–T2. However, the reduction of S. mutans counts in the probiotic group was significantly different during T2–T3 (p = 0.016; Table 1). The lactobacilli counts and buffer capacity of the probiotic group during T1–T2, T2–T3, and T1–T3 were not significantly different. The data was analyzed by Wilcoxon signed-rank test. (Table 1)
Within the control group, there were no significant differences in the S. mutans counts, lactobacilli counts and buffer capacity during T1–T2, T2–T3, and T1–T3. The data was analyzed by Wilcoxon signed-rank test. (Table 1)
The percentage of change in salivary S. mutans counts, lactobacilli counts, and buffer capacity was not significantly different in comparison with the probiotic group and control group during T1, T2, and T3. The data was analyzed by Fisher’s exact test. Although we could not find significant difference, the level of S. mutans counts (>105 CFU/ml, score 2 and 3) showed a trend for reduction during T2–T3 and a slight increment during T1–T2 (Fig. 2).
Percentage (95% confidence interval) of participants with high Streptococcus mutans counts (≥105 CFU/ml, score 2 and 3) in probiotic (n = 42) and control (n = 36) groups at baseline (T1), the completion of medication (T2), and 2 weeks after medication (T3). (Fisher’s exact test revealed p > 0.05 (NS))
Discussion
There were no significant differences in the S. mutans counts, lactobacilli counts, and buffer capacity between the probiotic and control groups at T1, T2, and T3. There were several possible reasons suspected for the nonsignificant difference in S. mutans counts between the probiotic and control groups. First, there was the relatively small sample size in our study. Second, the subjects only took L. paracasei for 2 weeks in our study. In Nase’s study, the intervention period of LGG was 7 months [2]. The 2-week intervention period might be too short to express differences between two groups. Further L. paracasei GMNL-33 studies with extended intervention time and increased subjects will be encouraged. In addition, the concentration and vehicle type of L. paracasei would not be efficient enough for reducing S. mutans rapidly.
Within the probiotic group, an interesting probiotic effect was noticed. Between T1 and T2, no inhibitory effect against S. mutans was observed. However, a significant count reduction in the salivary S. mutans was detected between T2 and T3 (p = 0.016). It seemed likely that once this probiotic had colonized into the oral cavity, it could exert a beneficial effect which did not occur during a short-term intervention. Thus, a 2-week period of medication via oral administration route may be needed for L. paracasei GMNL-33 to be effective in the probiotic action.
In this present study, salivary count of S. mutans within the probiotic group was significantly reduced only between T2 and T3 (p = 0.016). The S. mutans lowering effect of probiotics was more pronounced in the posttreatment period (T2-T3) rather than the intervention period (T1-T2). The result was comparable to the studies of Ahola [18] and Nikawa [6]. Ahola et al. [18] examined whether the short-term consumption of cheese containing LGG and L. rhamnosus LC 705 would diminish caries-associated salivary microbial counts in young adults. The results showed S. mutans counts were significantly reduced in the probiotic group, yet only during the posttreatment period. Nikawa et al. [6] examined the effect of short-term yogurt consumption with L. reuteri on the oral carriage of S. mutans. The results suggested that L. reuteri in yogurt reduced the S. mutans counts for up to 2 weeks after discontinuing the consumption. Both short-term probiotic interventions seemed to enhance the inhibitory effect against S. mutans after the intervention. Although we used different species of probiotic bacteria, the posttreatment effect was similar. But the mechanism of this posttreatment effect was not clear. Further studies for investigating the mechanism of the posttreatment effects of the probiotics would be needed.
Daily consumption of lactobacilli might lead to a transient (albeit permanent) colonization of these bacteria [19]. Busscher et al. [20] and Petti et al. [21] were unable to detect an oral colonization of lactobacilli after probiotic yogurt consumption. By contrast, Meurman et al. [22] reported that LGG was detected for up to 2 weeks after discontinued consumption of probiotic yogurt. Another bacteriocin producer S. salivarius K12 was used as a probiotic targeting the oral cavity. Horz et al. [23] monitored its dispersal and persistence in an oral environment by using real-time quantitative polymerase chain reaction. K12 could be detected at the mucosal membrane for 1 to 3 weeks yet with steadily decreasing tendency after 1 week. Thus probiotics may have the potential to control oral bacterial infections only when the uptake is repeated frequently.
We did not find statistically significant differences in the lactobacilli counts within and between probiotic versus control groups. However, there was a tendency for the probiotic intervention to increase the count levels of lactobacilli. Montalto investigated whether the oral administration of lactobacilli could change the salivary counts of these bacteria [24]. It was found that the oral administration of probiotics significantly increased salivary counts of lactobacilli. In another study, the salivary lactobacilli counts also increased after intake of the probiotic-containing cheese [18].
It should be noted that there were differences among various lactobacilli with regard to their abilities to cause dental caries. Some Lactobacillus species were identified in deep caries dentin and were related to dental caries progression. For example, Lactobacillus acidophilus and Lactobacillus casei Shirota might be strong agents in dental caries progression for their adhesive ability to dental surface [25]. However, it had been suggested that not all strains of Lactobacillus spp. had a caries-inducing effect [3]. In this study, the oral tablet contained L. paracasei extract that were not considered cariogenic. The elevation of count levels in lactobacilli could be reasonably considered as its well colonization in oral cavity under intervention instead of increasing caries risk [24].
Along with L. paracasei, the well-documented Lactobacillus GG was not considered cariogenic and had been shown to exert inhibitory activity against Streptococcus sobrinus at pH values below 5 [26]. The investigators also found that Lactobacillus GG did not ferment sucrose and thus did not promote caries. In addition, in vitro studies had shown that other lactobacilli, such as Lactobacillus fermentum and L. salivarius, had an inhibiting effect on the growth of S. mutans [27] and P. gingivalis [10]. All the lactobacilli except Lactobacillus jensenii produced bacteriocin against at least one of the indicator organisms. The ability of Lactobacillus spp. to protect their host against certain diseases by inhibiting the growth of potential pathogens was evident [15].
Salivary buffer capacity was not changed in the current L. paracasei GMNL-33 study. It was not changed on other comparable probiotic studies either [18].
In conclusion, the results of this study indicate that a 2-week period of medication at least may be needed for L. paracasei GMNL-33 via oral administration route to become effective in the probiotic action. And short-term probiotic intervention seemed to enhance its inhibitory effect against S. mutans after the intervention. Further studies for investigating the mechanism of the posttreatment effects of the probiotics would be needed.
References
Guarner F, Pedigon G, Coerthier G, Salminen S, Koletzko B, Morelli L (2005) Should yoghurt cultures be considered probiotic? Br J Nutr 93:783–786
Nase L, Hatakka K, Savilahti E, Saxelin M, Ponka A, Poussa T, Korpela R, Meurman JH (2001) Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res 35:412–420
Caglar E, Kavaloglu S, Ergeneli S, Sandalli N, Twetman S (2006) Salivary mutans streptococci and lactobacilli levels after ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol Scand 64:314–318
Caglar E, Kavaloglu SC, Kuscu OO, Sandalli N, Holgerson PL, Twetman S (2007) Effect of chewing gums containing xylitol or probiotic bacteria on salivary mutans streptococci and lactobacilli. Clin Oral Investig 11:425–429
Caglar E, Kuscu OO, Kavaloglu SC, Kuvvetli SS, Sandalli N (2008) A probiotic lozenge administered medical device and its effect on salivary mutans streptococci and lactobacilli. Int J Paediatr Dent 18:35–39
Nikawa H, Makihira S, Fukushima H, Nishimura H, Ozaki Y, Ishida K, Darmawan S, Hamada T, Hara K, Matsumoto T, Takemoto T, Aimi R (2004) Lactobacillus reuteri in bovine milk fermented decreases the oral carriage of mutans streptococci. Int J Food Microbiol 95:219–223
Caglar E, Sandalli N, Twetman S, Kavaloglu S, Ergeneli S, Selvi S (2005) Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol Scand 63:317–320
Caglar E, Kuscu OO, Kuvvetli SS, Kavaloglu SC, Sandalli N, Twetman S (2008) Short-term effect of ice-cream containing Bifidobacterium lactis Bb-12 on the number of salivary mutans streptococci and lactobacilli. Acta Odontol Scand 66:154–158
Hatakka K, Ahola AJ, Yli-Knuuttila H, Richardson M, Poussa T, Meurman JH, Korpela R (2007) Pobiotics reduce the prevalence of oral candida in the elderly—a randomized controlled trial. J Dent Res 86:125–130
Matsuoka T, Nakanishi M, Aiba Y, Koga Y (2004) Mechanism of Porphyromonas gingivalis killing by Lactobacillus salivarius TI 2711. J Jpn Soc Periodontol 46:118–126
Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G (2005) Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J 30:55–60
Kang MS, Kim BG, Chung J, Oh JS, Lee HC (2006) Inhibitory effect of Weissella cibaria isolates on the production of volatile sulphur compounds. J Clin Periodontol 33:226–232
Burton JP, Chilcott CN, Moore CJ, Speiser G, Tagg JR (2006) A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J Appl Microbiol 100:754–764
Sookkhee S, Chulasiri M, Prachyabrued W (2001) Lactic acid bacteria from healthy oral cavity of Thai volunteers: inhibition of oral pathogens. J Appl Microbiol 90:172–179
Kõll-Klais P, Mändar R, Leibur E, Marcotte H, Hammarström L, Mikelsaar M (2005) Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol 20:354–361
Simark-Mattsson C, Emilson C-G, Grahn Hakansson E, Jacobsson C, Roos K, Holm S (2007) Lactobacillus-mediated interference of mutans streptococci in caries-free vs. caries-active subjects. Eur J Oral Sci 115:308–314
Rosner B (2000) Fundamentals of biostatistics. Duxbury, Pacific Grove, p 410
Ahola AJ, Yli-Knuuttila H, Suomalainen T, Poussa T, Ahlstrom A, Meurman JH, Korpela R (2002) Short-term consumption of probiotic-containing cheese and its effect on dental caries risk factors. Arch Oral Biol 47:799–804
Teughels W, Van Essche M, Sliepen I, Quirynen M (2000) Probiotics and oral healthcare. Periodontol 48:111–147
Busscher HJ, Mulder AF, Van der Mei HC (1999) In vitro adhesion to enamel and in vivo colonization of tooth surfaces by Lactobacilli from a bio-yoghurt. Caries Res 33:403–404
Petti S, Tarsitani G, D’Arca AS (2001) A randomized clinical trial of effect of yoghurt on human salivary mocroflora. Arch Oral Biol 46:705–712
Meurman JH, Antila H, Salminen S (1994) Recovery of Lactobacillus strain GG (ATCC 53103) from saliva of healthy volunteers after consumption of yoghurt prepared with the bacterium. Micro Ecol Health Dis 7:295–298
Horz HP, Meinelt A, Houben B, Conrads G (2007) Distribution and persistence of probiotic Streptococcus salivarius K12 in the human oral cavity as determined by real-time quantitative polymerase chain reaction. Oral Microbiol Immunol 22:126–130
Montalto M, Vastola M, Marigo L, Covino M, Graziosetto R, Curigliano V, Santoro L (2004) Probiotic treatment increases salivary counts of lactobacilli: a double-blind, randomized, controlled study. Digestion 69:53–56
Lima LM, Motisuki C, Spolidorio DM, Santos-Pinto L (2005) In vitro evaluation of probiotics microorganisms adhesion to an artificial caries model. Eur J Clin Nutr 59:884–886
Meurman JH, Antila H, Korhonen A, Salminen S (1995) Effect of Lactobacillus rhamnosus strain GG (ATCC 53103) on the growth of Streptococcus sobrinus in vitro. Eur J Oral Sci 103:253–258
Ishihara K, Miyakawa H, Hasegawa A, Takazoe I, Kawai Y (1985) Growth inhibition of Streptococcus mutans by cellular extracts of human intestinal lactic acid bacteria. Infect Immun 49:692–694
Acknowledgements
We are grateful to residents, staff from the Department of Pediatric Dentistry, Division of Dentistry, Chang Gung Memorial Hospital at Linkou and Ms. Yu-Ting Liu and Hui Ou-Yang for their support in this study. We would like to express appreciation to Min-Chi Chen, Ph.D., for her assistance in the statistical analysis. We would like to extend our appreciation to the department heads, students of the Department of Nursing, and Department of Rehabilitation at College of Medicine, Chang Gung University, for their invaluable help and voluntary participation. This independent Chang Gung Research Project (XMRPG 460021) is funded by the GenMont Biotech Incorporation.
Conflict of interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Chuang, LC., Huang, CS., Ou-Yang, LW. et al. Probiotic Lactobacillus paracasei effect on cariogenic bacterial flora. Clin Oral Invest 15, 471–476 (2011). https://doi.org/10.1007/s00784-010-0423-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-010-0423-9