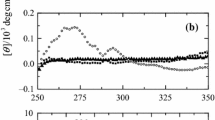
Abstract
Thermally denatured horse heart ferrocytochrome c (ferrocyt c) has been characterized using absorption spectroscopy, differential scanning calorimetry (DSC) and viscometry at pH 7.0. DSC experiments have yielded the transition temperature of denaturant-free ferrocyt c unfolding as 100.6±0.3 °C, indicating an extremely high stability of the protein. The presence of guanidine hydrochloride (GdnHCl) facilitated estimation of the structural features of thermally unfolded ferrocyt c. The stability of the protein, expressed by ΔG D at 25 °C, is 59±5 kJ mol−1 (DSC) and 65±6 kJ mol−1 (absorption spectroscopy). An absorption spectrum of ferrocyt c demonstrates that the heme occurs in the high-spin state at extreme denaturing conditions (94 °C, 6.6 M GdnHCl). Absorption spectroscopy, using heme as a probe, shows that thermal denaturation of ferrocyt c occurs as a transition from a native low-spin (Met80/His18) to a high-spin disordered state with involvement of non-native, low-spin (bis-His) species.










Similar content being viewed by others
Abbreviations
- CD:
-
circular dichroism
- cyt c :
-
cytochrome c
- DSC:
-
differential scanning calorimetry
- ferricyt c :
-
ferricytochrome c
- ferrocyt c :
-
ferrocytochrome c
- GdnHCl:
-
guanidine hydrochloride
- NHE:
-
normal hydrogen electrode
References
Moore GR, Pettigrew GW (1990) Cytochromes c: evolutionary, structural, and physicochemical aspects. Springer, New York
Dickerson RE, Takano T, Eisenberg D, Kallai OB, Samson L, Cooper A, Margoliash E (1971) J Biol Chem 246:1511–1535
Harbury HA, Cronin JR, Fanger MW, Hettinger TP, Murphy AJ, Myer YP, Vinogradov SN (1965) Proc Natl Acad Sci USA 54:1658–1664
Jeng MF, Englander W, Elöve GA, Wand AJ, Roder H (1990) Biochemistry 29:10433–10436
Moore GR, Williams RJL (1980) Eur J Biochem 103:523–532
Bixler J, Bakker G, McLendon G (1992) J Am Chem Soc 114:6938–6939
Yoshimura T (1988) Arch Biochem Biophys 264:450–461
Bhuyan AK, Udgaonkar JB (2001) J Mol Biol 312:1135–1160
Thomas YG, Goldbeck RA, Kliger DS (2000) Biopolymers 57:29–36
Pascher T, Chesick JP, Winkler JR, Gray HB (1996) Science 271:1558–1560
Goldbeck RA, Thomas YG, Chen E, Esquerra RM, Kliger DS (1999) Proc Natl Acad Sci USA 96:2782–2787
Luck SD, Bhuyan AK, Roder H (1993) Biophys J 64:A173–A173
McLendon G, Smith M (1978) J Biol Chem 253:4004–4008
Hickey DR, Berghuis AM, Lafond G, Jaeger JA, Cardillo TS, McLendon D, Das G, Sherman F, Brayer GD, McLendon G (1991) J Biol Chem 266:11686–11694
Roder H, Elöve GA, Englander SW (1988) Nature 335:700–704
Nall BT, Zuniga EH, White TB, Wood LC, Ramdas L (1989) Biochemistry 28:9834–9839
Tsong TY (1975) Biochemistry 14:1542–1547
Pace CN (1975) CRC Crit Rev Biochem 3:1–43
Schellman JA (1978) Biopolymers 17:1305–1322
Privalov PL (1992) In: Creighton TE (ed) Protein folding. Freeman, New York, pp 83–126
Pace CN (1986) Methods Enzymol 131:266–280
Santoro MM, Bolen DW (1992) Biochemistry 31:4901–4907
Sinha A, Yadav S, Ahmad R, Ahmad F (2000) Biochem J 345:711–717
Vickery L, Nozawa T, Sauer K (1976) J Am Chem Soc 98:351–357
Colón W, Wakem LP, Sherman F, Roder H (1997) Biochemistry 36:12535–12541
Elöve GA, Bhuyan AK, Roder H (1994) Biochemistry 33:6925–6935
Telford JR, Tezcan FA, Gray HB, Winkler JR (1999) Biochemistry 38:1944–1949
Taniguchi VT, Sailasuta-Scott N, Anson FC, Gray HB (1980) Pure Appl Chem 52:2275–2281
Cohen DS, Pielak GJ (1995) J Am Chem Soc 117:1675–1677
Acevedo O, Guzman-Casado M, Garcia-Mira MM, Ibarra-Molero B, Sanchez-Ruiz JM (2002) Anal Biochem 306:158–161
Margoliash E, Frohwirt N (1959) Biochem J 71:570–572
Kielly W, Harrington WF (1960) Biochim Biophys Acta 41:401–421
Santoro MM, Bolen DW (1988) Biochemistry 27:8063–8068
Sanchez-Ruiz JM, Lopez-Lacomba JL, Cortijo M, Mateo PL (1988) Biochemistry 27:1648–1652
Privalov PL, Khechinashvili NN (1974) J Mol Biol 86:665–684
Cohen DS, Pielak GJ (1994) Protein Sci 3:1253–1260
Perl D, Jacob M, Bánó M, Stupák M, Antalík M, Schmid FX (2002) Biophys Chem 96:173–190
Butt WD, Keilin D (1962) Proc Roy Soc B 156:429–457
Bae SJ, Sturtevant JM (1995) Biophys Chem 55:247–252
Mayr LM, Schmid FX (1993) Biochemistry 32:7994–7998
Schultes V, Jaenicke R (1991) FEBS Lett 290:235–238
Jones CM, Henry ER, Hu Y, Chan CK, Luck SD, Bhuyan A, Roder H, Hofrichter J, Eaton WA (1993) Proc Natl Acad Sci USA 90:11860–11864
Yoshimura T, Suzuki S, Nakahara A, Iwasaki H, Masuko M, Matsubara T (1985) Biochim Biophys Acta 831:267–274
Tsong TY (1974) J Biol Chem 249:1988–1990
Fisher WR, Taniuchi H, Anfinsen CA (1973) J Biol Chem 248:3188–3195
Stellwagen E (1967) J Biol Chem 242:602–606
Harding SE (1997) Prog Biophys Mol Biol 68:207–262
Doyle DF, Waldner JC, Purikh S, Alcazar-Roman L, Pielak GJ (1996) Biochemistry 35:7403–7411
Kretschmar M, Jaenicke R (1999) J Mol Biol 291:1147–1153
Hagihara Y, Tan Y, Goto Y (1994) J Mol Biol 237:336–348
Víglaský V, Antalík M, Bágel’ová J, Tomori Z, Podhradský D (2000) Electrophoresis 21:850–858
Robertson AD, Murphy KP (1997) Chem Rev 97:1251–1267
Pace CN (1990) Trends Biochem Sci 15:14–17
Privalov PL (1979) Adv Protein Chem 33:167–241
Dill KA (1990) Biochemistry 29:7133–7155
Bágel’ová J, Antalík M, Tomori Z (1997) Biochem Mol Biol Int 43:891–900
Moza B, Qureshi SH, Ahmad F (2003) Biochim Biophys Acta 1646:49–56
Berghuis AM, Brayer GD (1992) J Mol Biol 223:959–976
Tezcan FA, Winkler JR, Gray HB (1998) J Am Chem Soc 120:13383–13388
Stellwagen E (1968) Biochemistry 7:2893–2897
Ahmad Z, Ahmad F (1992) Indian J Chem B 31:874–879
Tanford C, Kawahara K, Lapanje S (1967) J Am Chem Soc 89:729–736
Babul J, Stellwagen E (1971) Biopolymers 10:2359–2361
Muthukrishnan K, Nall BT (1991) Biochemistry 30:4706–4710
Godbole S, Bowler BE (1997) J Mol Biol 268:816–821
Banci L, Bertini I, Spyroulias GA, Turano P (1998) Eur J Inorg Chem 583–591
Filosa A, English AM (2000) J Biol Inorg Chem 5:448–454
Lu J, Liu G, Chen Y, Tang W, Zhu D (1998) Inorg Chim Acta 275–276:58–64
Dopner S, Hildebrandt P, Rosell FI, Mauk AG (1998) J Am Chem Soc 120:11246–11255
Ferrer JC, Guillemette JG, Bogumil R, Inglis SC, Smith M, Mauk AG (1993) J Am Chem Soc 115:7507–7508
Liu G, Chen Y, Tang W (1997) J Chem Soc Dalton Trans 795–802
Russell BS, Bren KL (2002) J Biol Inorg Chem 7:909–916
Russell BS, Melenkivitz R, Bren KL (2000) Proc Natl Acad Sci USA 97:8312–8317
Meyer TE, Kamen MD (1982) Adv Protein Chem 35:105–212
Finzel BC, Weber PC, Hardman KD, Salemme FR (1985) J Mol Biol 186:627–643
Yoshimura T, Fujii S, Kamada H, Yamaguchi K, Suzuki S, Shidara S, Takakuwa S (1996) Biochim Biophys Acta 1292:39–46
McCrary BS, Edmondson SP, Shriver JW (1996) J Mol Biol 264:784–805
Nojima H, Ikai A, Oshima T, Noda H (1977) J Mol Biol 116:429–442
Klump H, Ruggiero J, Kessel M, Park JB, Adams MWW, Robb F (1992) J Biol Chem 267:22681–22685
Acknowledgements
This work was supported by a research grant from the Slovak Grant Agency VEGA no. 3198. We thank Drs. Jaroslava Bágel’ová and Erik Sedlák for helpful comments, and a reviewer for correcting the English.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Varhač, R., Antalík, M. & Bánó, M. Effect of temperature and guanidine hydrochloride on ferrocytochrome c at neutral pH. J Biol Inorg Chem 9, 12–22 (2004). https://doi.org/10.1007/s00775-003-0492-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-003-0492-1