Abstract
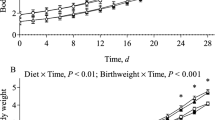
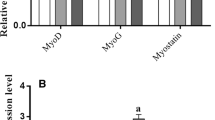
The objective of this study was to determine if enteral leucine or branched-chain amino acid (BCAA) supplementation increases muscle protein synthesis in neonates who consume less than their protein and energy requirements, and whether this increase is mediated via the upregulation of the mechanistic target of rapamycin complex 1 (mTORC1) pathway or the decrease in muscle protein degradation signaling. Neonatal pigs were fed milk replacement diets containing reduced energy and protein (R), R supplemented with BCAA (RBCAA), R supplemented with leucine (RL), or complete protein and energy (CON) at 4-h intervals for 9 (n = 24) or 21 days (n = 22). On days 9 and 21, post-prandial plasma amino acids and insulin were measured at intervals for 4 h; muscle protein synthesis rate and activation of mTOR-related proteins were determined at 120 min post-feeding in muscle. For all parameters measured, the effects of diet were not different between day 9 or day 21. Compared to CON and R, plasma leucine and BCAA were higher (P ≤ 0.01) in RL- and RBCAA-fed pigs, respectively. Body weight gain, protein synthesis, and activation of S6 kinase (S6K1), 4E-binding protein (4EBP1), and eukaryotic initiation factor 4 complex (eIF4E·eIF4G) were decreased in RBCAA, RL, and R relative to CON (P < 0.01). RBCAA and RL upregulated (P ≤ 0.01) S6K1, 4EBP1, and eIF4E·eIF4G compared to R. In conclusion, when protein and energy are restricted, both leucine and BCAA supplementation increase mTOR activation, but do not enhance skeletal muscle protein synthesis and muscle growth in neonatal pigs.








Similar content being viewed by others
Abbreviations
- AA:
-
Amino acids
- AMPKα:
-
AMP-activated protein kinase α
- AU:
-
Arbitrary units
- BCAA:
-
Branched-chain amino acids
- BW:
-
Body weight
- CON:
-
Control
- CP:
-
Crude protein
- C S :
-
Muscle protein synthetic capacity
- DXA:
-
Dual-energy X-ray absorptiometry
- EAA:
-
Essential amino acid
- eIF4E eIF4G:
-
Eukaryotic initiation factor 4 complex
- ERK1/2:
-
Extracellular signal-regulated kinases 1 and 2
- 4EBP1:
-
Eukaryotic initiation factor repressor 4E-binding protein 1
- FOXO1:
-
Forkhead box protein O1
- HPLC:
-
High-performance liquid chromatography
- HOMA:
-
Homeostatic model assessment
- IRS1:
-
Insulin receptor substrate 1
- K RNA :
-
Protein synthesis efficiency
- K S :
-
Fractional rate of protein synthesis
- LAT:
-
l-Type amino acid transporter 1
- LBW:
-
Low birthweight
- LD:
-
Longissimus dorsi
- MAFbx:
-
Muscle atrophy F-Box/Atrogin-1
- ME:
-
Metabolizable energy
- mTORC1:
-
Mechanistic target of rapamycin complex 1
- MuRF1:
-
Muscle RING-finger protein-1
- NEAA:
-
Non-essential amino acids
- PKB/Akt:
-
Protein kinase B
- P/T:
-
Phosphorylated/total protein
- R:
-
Restricted
- RL:
-
Restricted supplemented with leucine
- RBCAA:
-
Restricted supplemented with BCAA
- S6K1:
-
Ribosomal protein S6 kinase 1
- SNAT:
-
Na+-coupled neutral amino acid transporter 2
- TSC2:
-
Tuberous sclerosis complex 2
References
Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR (2000) Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr 130:2413–2419
Ariano MA, Armstrong RB, Edgerton VR (1973) Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem 21:51–55
Atlantis E, Martin SA, Haren MT, Taylor AW, Wittert GA (2009) Inverse associations between muscle mass, strength, and the metabolic syndrome. Metabolism 58:1013–1022
Ban H, Shigemitsu K, Yamatsuji T, Haisa M, Nakajo T, Takaoka M, Nobuhisa T, Gunduz M, Tanaka N, Naomoto Y (2004) Arginine and leucine regulate p70S6 kinase and 4E-BP1 in intestinal epithelial cells. Int J Mol Med 13:537–543
Berseth CL (2001) Feeding methods for the preterm infant. Semin Neonatol 6:417–424
Berseth CL (2005) Feeding strategies and necrotizing enterocolitis. Curr Opin Pediatr 17:170–173
Black JL, Griffiths DA (1975) Effects of live weight and energy intake on nitrogen balance and total N requirement of lambs. Br J Nutr 33:399–413
Boutry C, El-Kadi SW, Suryawan A, Wheatley SM, Orellana RA, Kimball SR, Nguyen HV, Davis TA (2013) Leucine pulses enhance skeletal muscle protein synthesis during continuous feeding in neonatal pigs. Am J Physiol Endocrinol Metab 305:E620–E631
Boutry C, El-Kadi SW, Suryawan A, Steinhoff-Wagner J, Stoll B, Orellana RA, Nguyen HV, Kimball SR, Fiorotto ML, Davis TA (2016) Pulsatile delivery of a leucine supplement during long-term continuous enteral feeding enhances lean growth in term neonatal pigs. Am J Physiol Endocrinol Metab 310:E699–E713
Brown SM, Klaffenbock M, Nevison IM, Lawrence AB (2015) Evidence for litter differences in play behavior in pre-weaned pigs. Appl Anim Behav Sci 172:17–25
Burrin DG, Davis TA, Ebner S, Schoknecht PA, Fiorotto ML, Reeds PJ, McAvoy S (1995) Nutrient-independent and nutrient-dependent factors stimulate protein synthesis in colostrum-fed newborn pigs. Pediatr Res 37:593–599
Campbell RG, Dunkin AC (1983) The effects of energy intake and dietary protein on nitrogen retention, growth performance, body composition and some aspects of energy metabolism of baby pigs. Br J Nutr 49:221–230
Churchward-Venne TA, Burd NA, Mitchell CJ, West DW, Philp A, Marcotte GR, Baker SK, Baar K, Phillips SM (2012) Supplementation of a suboptimal protein dose with leucine or essential amino acids: effects on myofibrillar protein synthesis at rest and following resistance exercise in men. J Physiol 590:2751–2765
Columbus DA, Steinhoff-Wagner J, Suryawan A, Nguyen HV, Hernandez-Garcia A, Fiorotto ML, Davis TA (2015) Impact of prolonged leucine supplementation on protein synthesis and lean growth in neonatal pigs. Am J Physiol Endocrinol Metab 309:E601–E610
Davis TA, Fiorotto ML, Nguyen HV, Reeds PJ (1989) Protein turnover in skeletal muscle of suckling rats. Am J Physiol 257:R1141–R1146
Davis TA, Burrin DG, Fiorotto ML, Nguyen HV (1996) Protein synthesis in skeletal muscle and jejunum is more responsive to feeding in 7- than in 26-day-old pigs. Am J Physiol 270:E802–E809
Davis TA, Fiorotto ML, Nguyen HV, Burrin DG (1999) Aminoacyl-tRNA and tissue free amino acid pools are equilibrated after a flooding dose of phenylalanine. Am J Physiol 277:E103–E109
Davis TA, Nguyen HV, Suryawan A, Bush JA, Jefferson LS, Kimball SR (2000) Developmental changes in the feeding-induced stimulation of translation initiation in muscle of neonatal pigs. Am J Physiol Endocrinol Metab 279:E1226–E1234
Davis TA, Fiorotto ML, Burrin DG, Reeds PJ, Nguyen HV, Beckett PR, Vann RC, O’Connor PM (2002) Stimulation of protein synthesis by both insulin and amino acids is unique to skeletal muscle in neonatal pigs. Am J Physiol Endocrinol Metab 282:E880–E890
De Curtis M, Rigo J (2004) Extrauterine growth restriction in very-low-birthweight infants. Acta Pediatr 93:1563–1568
Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB (2010) An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298:E1011–E1018
Duffy B, Gunn T, Collinge J, Pencharz P (1981) The effect of varying protein quality and energy intake on the nitrogen metabolism of parenterally fed very low birthweight (less than 1600 g) infants. Pediatr Res 15:1040–1044
Ehrenkranz RA (2007) Early, aggressive nutritional management for very low birth weight infants: what is the evidence? Semin Perinatol 31:48–55
Embleton NE, Pang N, Cooke RJ (2001) Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? J Pediatr 107:270–273
Escobar J, Frank JW, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2005) Physiological rise in plasma leucine stimulates muscle protein synthesis in neonatal pigs by enhancing translation initiation factor activation. Am J Physiol Endocrinol Metab 288:E914–E921
Escobar J, Frank JW, Suryawan A, Nguyen HV, Davis TA (2007) Amino acid availability and age affect the leucine stimulation of protein synthesis and eIF4F formation in muscle. Am J Physiol Endocrinol Metab 293:E1615–E1621
Fiorotto ML, Davis TA, Reeds PJ, Burrin DG (2000) Nonnutritive factors in colostrum enhance myofibrillar protein synthesis in the newborn pig. Pediatr Res 48:1–7
Gosmanov AR, Nodtredt NC, Brown R, Thomason DB (1985) Exercise effects on muscle beta-adrenergic signaling for MAPK-dependent NKCC activity are rapid and persistent. J Appl Physiol 93:1457–1465
Harper AE, Benevenga NJ, Wohlhueter RM (1970) Effects of ingestion disproportionate amounts of amino acids. Physiol Rev 50:428–558
Hay WW (2008) Strategies for feeding the preterm infant. Neonatology 94:245–254
Hernandez-García AD, Columbus DA, Manjarín R, Nguyen HV, Suryawan A, Orellana RA, Davis TA (2016) Leucine supplementation stimulates protein synthesis and reduces degradation signal activation in muscle of newborn pigs during acute endotoxemia. Am J Physiol Endocrinol Metab 311:E791–E801
Hundal HS, Taylor PM (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296:E603–E613
Iwanaka N, Egawa T, Satoubu N, Karaike K, Ma X, Masuda S, Hayashi T (1985) Leucine modulates contraction- and insulin-stimulated glucose transport and upstream signaling events in rat skeletal muscle. J Appl Physiol 108:274–282
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2000) LC3, a mammalian holomogue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Kelly FJ, Lewis SE, Anderson P, Goldspink DF (1984) Pre- and postnatal growth and protein turnover in four muscles of the rat. Muscle Nerve 7:235–242
Kim J, Song G, Wu G, Gao H, Johnson GA, Bazer FW (2013) Arginine, leucine, and glutamine stimulate proliferation of porcine trophectoderm cells through the MTOR-RPS6K-RPS6-EIF4EBP1 signal transduction pathway. Biol Reprod 88:113–121
Koo WW, Hockman EM, Hammami M (2004) Dual energy X-ray absorptiometry measurements in small subjects: conditions affecting clinical measurements. J Am Coll Nutr 23(3):212–219
Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH (2007) AMP-activated protein kinase agonists increase mRNA content of the muscle specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292:E1555–E1567
Kumar RK, Singhal A, Vaidya U, Banerjee S, Anwar F, Rao S (2017) Optimizing nutrition in preterm low birth weight infants-consensus summary. Front Nutr 4:20
Lapillonne A, Griffin IJ (2013) Feeding preterm infants today for later metabolic and cardiovascular outcomes. J Pediatr 162(3 Suppl):S7–S16
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Manjarín R, Columbus DA, Suryawan A, Nguyen HV, Hernandez-García AD, Hoang NM, Fiorotto ML, Davis T (2016) Leucine supplementation of a restricted protein and energy diet enhances mTORC1 activation but not muscle protein synthesis in neonatal pigs. Amino Acids 48:257–267
March of Dimes Foundation (2014). http://www.marchofdimes.org/mission/prematurity-campaign.aspx. Accessed 6 Oct 2014
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
McCubrey JA, Steelman LS, Kempf CR, Chappell WH, Abrams SL, Stivala F, Malaponte G, Nicoletti F, Libra M, Basecke J, Maksimovic-Ivanic D, Mijatovic S, Montalto G, Cervello M, Cocco L, Martelli AM (2011) Therapeutic resistance resulting from mutations in Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR signaling pathways. J Cell Physiol 226:2762–2781
Mourier A, Bigard AX, Kerviler E, Roger B, Legrand H, Guezennec CY (1997) Combined effects of caloric restriction and branched chain amino acid supplementation on body composition and exercise performance in elite wrestlers. Int J Sports Med 18:47–55
Munro N, Fleck A (1966) The determination of nucleic acids. Meth Biochem Anal 14:113–176
Nadeau KJ, Ehlers LB, Aguirre LE, Moore RL, Korinne NJ, Ortmeyer HK, Hansen BC, Reusch JEB, Draznin B (2006) Exercise training and calorie restriction increase SREBP-1 expression in intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab 291:E90–E98
National Research Council (2012) Nutrient requirements of swine, 11th rev ed. National Academy Press, Washington (DC)
Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL 3rd, Goodyear LJ, Tong Q (2009) Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 1:771–783
Pasiakos SM, McClung HL, McClung JP, Margolis LM, Andersen NE, Cloutier GJ, Pikosky MA, Rood JC, Fielding RA, Young AJ (2011) Leucine-enriched essential amino acid supplementation during moderate steady state exercise enhances postexercise muscle protein synthesis. Am J Clin Nutr 94:809–818
Piepho HP (2009) Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101:865–869
Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D (2006) Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol 575:305–315
Rigo J, De Curtis M, Pieltain C (2002) Nutritional assessment and body composition of preterm infants. Semin Neonatol 6:383–391
Ryu JM, Han HJ (2011) L-threonine regulates G1/S phase transition of mouse embryonic stem cells via PI3K/Akt, MAPKs, and mTORC pathways. J Biol Chem 286:23667–23678
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
Suryawan A, Nguyen HV, Bush JA, Davis TA (2001) Developmental changes in the feeding-induced activation of the insulin-signaling pathway in neonatal pigs. Am J Physiol Endocrinol Metab 281:E908–E915
Suryawan A, Torrazza RM, Gazzaneo MC, Orellana RA, Fiorotto ML, El-Kadi SW, Srivastava N, Nguyen HV, Davis TA (2012) Enteral leucine supplementation increases protein synthesis in skeletal and cardiac muscles and visceral tissues of neonatal pigs through mTORC1-dependent pathways. Pediatr Res 71:324–331
Tannous RE, Rogers QR, Harper AE (1963) Effect of leucine-isoleucine and valine antagonism on the pattern of free amino acids in blood plasma and several tissues of the rat. Fed Proc 22:202–210
Torrazza RM, Suryawan A, Gazzaneo MC, Orellana RA, Frank JW, Nguyen HV, Fiorotto ML, El-Kadi S, Davis TA (2010) Leucine supplementation of a low-protein meal increases skeletal muscle and visceral tissue protein synthesis in neonatal pigs by stimulating mTOR-dependent translation initiation. J Nutr 140:2145–2152
Tremblay F, Brûlé S, Um SH, Li Y, Masuda K, Roden M, Sun XJ, Krebs M, Polakiewicz RD, Thomas G, Marette A (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA 104:14056–14061
Wang Ch, Wang Y, McNutt M, Zhu W (2011) Autophagy process is associated with anti-neoplastic function. Acta Biochim Biophys Sin 43:425–432
Whincup PH, Kaye SJ, Owen CG, Huxley R et al (2008) Birth weight and risk of type 2 diabetes: a systematic review. J Am Med Assoc 300:2886–2897
Wilson FA, Suryawan A, Gazzaneo MC, Orellana RA, Nguyen HV, Davis TA (2010) Stimulation of muscle protein synthesis by prolonged parenteral infusion of leucine is dependent on amino acid availability in neonatal pigs. J Nutr 140:264–270
Wray-Cahen D, Nguyen HV, Burrin DG, Beckett PR, Fiorotto ML, Reeds PJ, Wester TJ, Davis TA (1998) Response of skeletal muscle protein synthesis to insulin in suckling pigs decreases with development. Am J Physiol Endocrinol Metab 275:E602–E609
Yin YL, Yao K, Liu ZJ, Gong M, Ruan Z, Deng D, Tan BE, Liu ZQ, Wu G (2010) Supplementing l-leucine to a low-protein diet increases tissue protein synthesis in weanling pigs. Amino Acids 39:1477–1486
Zhang S, Qiao S, Ren M, Zeng X, Ma X, Wu Z, Thacker P, Wu G (2013) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45:1191–1205
Ziegler EE, Thureen PJ, Carlson SJ (2002) Aggressive nutrition of very low birthweight infant. Clin Perinatol 29:225–244
Acknowledgements
The work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases Grants AR-044474 (Davis) and AR-46308 (Fiorotto), the National Institute of Child Health and Human Development HD-072891 and HD085573 (Davis), United States Department of Agriculture National Institute of Food and Agriculture Grant 2013-67015-20438 (Davis), California State University Agricultural Research Institute Grant 58982 (Manjarin), and by the USDA/ARS under Cooperative Agreement No. 6250-510000-055 (Davis). This work is a publication of the USDA, Agricultural Research Service (USDA/ARS) Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX. The contents of this publication do not necessarily reflect the views or politics of the USDA, nor do the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving animals
Experiments were carried out in accordance with the Institutional Animal Care and Use Committee of Baylor College of Medicine and conducted in accordance with the National Research Council´s Guide for the Care and Use of Laboratory Animals (2011).
Informed consent
Not applicable (no human subjects were used in this study).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Manjarín, R., Columbus, D.A., Solis, J. et al. Short- and long-term effects of leucine and branched-chain amino acid supplementation of a protein- and energy-reduced diet on muscle protein metabolism in neonatal pigs. Amino Acids 50, 943–959 (2018). https://doi.org/10.1007/s00726-018-2572-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2572-0