Abstract
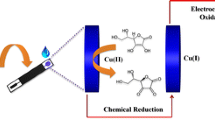
We describe a composite material for use in electrochemical oxygen reduction. A screen-printed electrode (SPE) was consecutively modified with electrodeposited copper, a Nafion membrane and electropolymerized polyaniline (PANi) to give an electrocatalytic composite of type PANi/Nafion/Cu2O/SPE that displays good electrical conductivity at neutral pH values. It is found that the presence of ammonia causes complex formation with Cu(I), and this causes electroreduction of oxygen to result in an increased cathodic current. The finding was applied to the quantification of ammonium ions in the 1 to 1000 μM concentration range by amperometry at −0.45 V (vs. Ag/AgCl). This Faradaic phenomenon offers the advantage of direct voltammetric detection, one of the lowest known limits of detection (0.5 μM), and high sensitivity (250 mA∙M−1∙cm−2). It was applied to the determination of ammonium ion in human serum where it compared well with the photometric routine approach for clinical analysis using glutamate dehydrogenase.

An electrocatalytic material for oxygen reduction, based on polyaniline in combination with copper, is described. It is found that the presence of ammonia causes complex formation with copper, and this causes electroreduction of oxygen to result in an increased current. This phenomenon offers the advantage of direct detection, one of the lowest known limits of detection and high sensitivity, which was utilized for ammonium ion determination in human serum.




Similar content being viewed by others
References
Abass AK, Hart JP, Cowell DC, Chappell A (1998) Development of an amperometric assay for NH4+ based on a chemically modified screen-printed NADH sensor. Anal Chim Acta 373:1–8
Annamalai SK, Palani B, Pillai KC (2012) Highly stable and redox active nano copper species stbilized functionalized-multiwalled carbon nanotube/chitosan modified electrode for efficient hydrogen peroxide reduction. Colloids Surf A Physicochem Eng Asp 395:207–216
Bertocchi P, Compagnone D, Palleschi G (1996) Amperometric ammonium ion and urea determination with enzyme-based probes. Biosensors & Bioelectronics 11:1–10
Burke LD, Ahern MJG, Ryan TG (1990) An investigation of the anodic behaviour of copper and its anodically produced oxides in aqueous solutions of high pH. J Electrochem Soc 137:553–561
Cho WJ, Huang HJ (1998) An amperometric urea biosensor based on a polyaniline-perfluorosulfonated ionomer composite electrode. Anal Chem 70:3946–3951
Davis J, Moorcroft MJ, Wilkins SJ, Compton RG, Cardosi MF (2000) Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 125:737–741
Deng AP, Cheng JT, Huang HJ (2002) Application of a polyaniline based ammonium sensor for the amperometric immunoassay of a urease conjugated Tal 1 protein. Anal Chim Acta 461:49–55
Fogg AG, Scullion SP, Edmonds TE, Birch BJ (1991) Direct reductive amperometric determination of nitrate at a copper electrode formed ln situ In a capillary-fill sensor device. Analyst 116:573–579
Giannopoulou I, Panias D, Paspaliaris I (2009) Electrochemical modeling and study of copper deposition from concentrated ammoniacal sulfate solutions. Hydrometallurgy 99:58–66
Hamlaoui ML, Kherrat R, Marrakchi M, Jaffrezic-Renault N, Walcarius A (2002) Development of an ammonium ISFET sensor with a polymeric membrane including zeolite. Materials Science & Engineering C-Biomimetic And Supramolecular Systems 21:25–28
Hamlaoui ML, Reybier K, Marrakchi M, Jaffrezic-Renault N, Martelet C, Kherrat R, Walcarius A (2002) Development of a urea biosensor based on a polymeric membrane including zeolite. Anal Chim Acta 466:39–45
Heng LY, Alva S, Ahmad M (2004) Ammonium ion sensor based on photocured and self-plasticising acrylic films for the analysis of sewage. Sensors and Actuators B-Chemical 98:160–165
Hirai T, Kuwabata S, Yoneyama H (1988) Electrochemical behaviors of polypyrrole, poly-3-methylthyophene and polyaniline deposited on nafion-coated electrodes. J Electrochem Soc 135:1132–1137
Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin JS, Duffy TE, Plum F (1979) Dynamics of ammonia metabolism in man - effects of liver-disease and hyper-ammonemia. J Clin Investig 63:449–460
Luo YC, Do JS (2004) Urea biosensor based on PANi(urease)-Nation (R)/Au composite electrode. Biosensors & Bioelectronics 20:15–23
Nagasubramanian G, Distefano S, Moacanin J (1986) Electrochemical incorporation of poly(pyrrole) into nafion and comparison of the electrochemical properties of nafion poly(pyrrole) Amd poly(pyrrole) film. J Phys Chem 90:4447–4451
Nicholls D (1973) Complexes and first-Row transition elements. Macmillan Press, London
Ribeiro JA, Silva F, Pereira CM (2012) Electrochemical sensing of ammonium ion at the water/1,6-dichlorohexane interface. Talanta 88:54–60
Shin JH, Yoon SY, Yoon IJ, Choi SH, Lee SD, Nam H, Cha GS (1998) Potentiometric biosensors using immobilized enzyme layers mixed with hydrophilic polyurethane. Sensors and Actuators B-Chemical 50:19–26
Silva R, Voiry D, Chhowalla M, Asefa T (2013) Efficient metal-free electrocatalysts for oxygen reduction: polyaniline-derived N- and O-doped mesoporous carbons. JACS 135:7823–7826
Stasyuk N, Smutok O, Gayda G, Vus B, Koval'chuk Y, Gonchar M (2012) Bi-enzyme L-arginine-selective amperometric biosensor based on ammonium-sensing polyaniline-modified electrode. Biosensors & Bioelectronics 37:46–52
Strehlitz B, Grundig B, Kopinke H (2000) Sensor for amperometric determination of ammonia and ammonia-forming enzyme reactions. Anal Chim Acta 403:11–23
Thompson M, Krull UJ, Bendellyoung LI (1983) The bilayer lipid-membrane as a basis for a selective sensor for ammonia. Talanta 30:919–924
Vanson M, Schothorst RC, Denboef G (1983) Determination of total ammoniacal nitrogen in water by flow-injection analysis and a gas-diffusion membrane. Anal Chim Acta 153:271–275
Vazquez-Arenas J, Lazaro I, Cruz R (2007) Electrochemical study of binary and ternary copper complexes in ammonia-chloride medium. Electrochim Acta 52:6106–6117
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman D (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Whittingham MS, Zawodzinski T (2004) Introduction: batteries and fuel cells. Chem Rev 104:4243
Winther-Jensen B, Winther-Jensen O, Forsyth M, Macfarlane DR (2008) High rates of oxygen reduction over a vapor phase-polymerized PEDOT electorde. Science 321:671
Yuan Y, Ahmed J, Kim S (2011) Polyaniline/carbon black composite-supported iron phtalocyanine as an oxygen reduction catalyst for microbial fuel cell. J Power Sources 196:1103
Acknowledgments
This work was partially funded by the “SMARTCANCERSENS” project from the European Community Seventh Framework Program under the Grant Agreement PIRSES-GA-2012-318053 and by the NATO Science for Peace (SFP) Project CBP.NUKR.SFPP 984173. Authors would like to thank also Dr. Olga Beloivan and Mr. Krishnakumar Pillai for SEM and EDX measurements.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
ESM 1
(DOCX 1305 kb)
Rights and permissions
About this article
Cite this article
Zhybak, M.T., Vagin, M.Y., Beni, V. et al. Direct detection of ammonium ion by means of oxygen electrocatalysis at a copper-polyaniline composite on a screen-printed electrode. Microchim Acta 183, 1981–1987 (2016). https://doi.org/10.1007/s00604-016-1834-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1834-3