Abstract
Purpose
Patients undergoing extensive cervical spine surgery (ECSS) occasionally require emergency reintubation due to postoperative airway complications. To avoid it, an endotracheal tube is retained in patients maintained under sedation overnight. This study was conducted to determine whether dexmedetomidine would be superior in sedative effects to propofol for postoperative sedation after ECSS.
Methods
We studied 32 consecutive patients undergoing ECSS who required prophylactic intubation postoperatively under sedation overnight. The patients were randomly divided into two groups. Group D (n = 16) received dexmedetomidine 0.1 μg/kg/min for 10 min as a loading dose, followed by a continuous infusion at 0.4 μg/kg/h. Group P (n = 16) received propofol 0.1 mg/kg/min for 10 min as a loading dose, followed by a continuous infusion at 1 mg/kg/h. All patients received analgesia with buprenorphine. Ramsay sedation scale, extremity movement, and pain intensity were recorded every 2 h. Dexmedetomidine and propofol dosages were adjusted to maintain a desired sedation level. Nursing staff adjusted dopamine to maintain systolic blood pressure >100 mmHg and administered atropine when the heart rate was <50 bpm.
Results
The proportions of adequate sedation level, movement, and pain status were similar between groups. In group D, heart rates were lower, frequency of atropine use was greater, and dopamine dose was higher than in group P.
Conclusion
Both sedatives are efficacious after ECSS; however, dexmedetomidine decreased heart rate and required higher dose of dopamine.
Similar content being viewed by others
Introduction
Patients undergoing extensive cervical spine surgery (ECSS) occasionally require postoperative emergency reintubation or tracheotomy due to postoperative airway complications [1]. It is difficult to perform reintubation because of pharyngeal swelling and contraindicated neck hyperextension [2]. Moreover, emergency reintubation without muscle relaxant is a risk factor for postintubation laryngeal injury [3]. To avoid this procedure, an endotracheal tube is retained in patients maintained under sedation overnight after ECSS, and evaluation of the upper airway status is performed prior to extubation on the first postoperative day [1, 4]. However, sedation after ECSS is required to enable the patient to move his/her extremities in response to verbal commands to determine nerve paralysis associated with postoperative hematoma. Sedation with dexmedetomidine has properties similar to natural sleep, and patients lightly sedated with dexmedetomidine are easily aroused and responsive to verbal commands [5, 6]. Thus, dexmedetomidine seems to be an ideal sedative for postoperative sedation after ECSS. This randomized, open-label, clinical study was carried out to compare the sedative effects of dexmedetomidine and propofol for postoperative sedation after ECSS.
Materials and methods
Patients
This study was approved by the Institutional Ethics Committee and conducted in the intensive care unit (ICU) of Nagasaki Rosai Hospital from September 2005 to August 2009. Written informed consent was obtained from each patient. We studied 32 consecutive patients undergoing ECSS who required postoperative endotracheal intubation and mechanical ventilation under sedation overnight. Patients with preoperative endotracheal intubation and those younger than 18 years of age were excluded from the study. Preoperative neuroradiographic studies included myelography, computed axial tomographic myelography, and magnetic resonance imaging. These studies revealed the extent of spinal cord compression and were used to determine the type and extent of the planned operative procedure.
Operative and anesthetic procedure
In patients with gross instability or in whom hyperextension was contraindicated, fiberoptic awake intubation was performed prior to inducing anesthesia. Otherwise, standard endotracheal intubation was performed via direct laryngoscopy after the induction of anesthesia. Anesthesia was induced with thiamylal or propofol and maintained with sevoflurane. Fentanyl was used as the analgesic agent, and controlled ventilation was maintained with vecuronium i.v. during the operation. An extensive anterior, posterior, or combined anterior–posterior procedure was performed by three regular orthopedists. The anterior procedure included more than three levels of cervical discectomy or corpectomy with bone graft fusion. The posterior procedure included more than three levels of cervical laminectomy and posterior fusion, including, if necessary, the occipital bone or thoracic spinal bone. The combined anterior–posterior procedure consisted of a cervical discectomy or multilevel anterior cervical discectomy or corpectomy with bone graft fusion and a multilevel cervical laminoplasty or posterior fusion. The wound was closed in a standard manner using closed suction.
Sedation
Patients were transported from the operating theater to the ICU after the operation. Anesthesia was maintained with sevoflurane until just before transportation, and muscle relaxant was reversed with neostigmine after the operation. The patients’ tracheas were kept intubated. All patients received analgesia with bolus of buprenorphine 4 μg/kg at the end of anesthesia, followed by a continuous infusion at a fixed dose of 0.3 μg/kg/h at admission to ICU. We maintained continuous infusion of buprenorphine during study period. Patients were randomly divided by sealed envelope assignment into two groups (D and P) just prior to transportation from the operating theater. Patients received hemodynamic monitoring consisting of continuous recordings of electrocardiogram (ECG), heart rate, and arterial blood pressure. The ability of patients to move their extremities in response to verbal commands was confirmed after arousal from anesthesia in the ICU, and then the study was started. Group D (n = 16) received dexmedetomidine at 0.1 μg/kg/min for 10 min as a loading dose, followed by a continuous infusion at 0.4 μg/kg/h. Group P (n = 16) received propofol at 0.1 mg/kg/min for 10 min as a loading dose, followed by a continuous infusion at 1 mg/kg/h. The Ramsay sedation scale (RSS) was recorded by the nursing staff every 2 h. Doses were adjusted to maintain the desired sedation at 2, 3, or 4 on the RSS by a 0.1 μg/kg/h increase or decrease of dexmedetomidine in group D, and a 0.3 mg/kg/h increase or decrease of propofol in group P. A bolus of either 1 mg/kg propofol or 0.1 μg/kg dexmedetomidine was added according to the clinical indication. If adequate sedation was not achieved at maximum infusion rate (1 μg/kg/min in group D and 3 mg/kg/h in group P), another sedative, as additional use, was administered at initial continuous infusion rate.
Study procedure
Patients were breathing spontaneously using the pressure support mode under sedation. The attending physicians adjusted the ventilator settings to maintain clinically appropriate gas exchange. In addition to RSS evaluation, heart rate, blood pressure, movement of extremities, pain intensity, urine output, and minute ventilation were simultaneously evaluated by nursing staff every 2 h. Movement of extremities was classified as follows: 2, response to verbal commands; 1, response to a light tap; and 0, no response. Adequate movement was determined by a classification of 2. Pain intensity was evaluated on a verbal rating scale of 0 (none) to 2 (severe). Adequate pain status was defined by a score of 0. Patients’ tracheas were kept intubated postoperatively under sedation overnight. Evaluation of the neck and pharynx was performed the following morning (9–10 a.m.), when the study was discontinued. The study period was set from the time of administering the loading dose of a sedative to the evaluation of the neck and pharynx. When the evaluation ruled out postoperative reactive swelling of the neck and pharynx, infusion of the study drug was discontinued. Tracheas were extubated when the patients recovered a normal level of consciousness. On the other hand, prophylactic delayed extubation under sedation was performed if the evaluation revealed postoperative swelling of the neck and pharynx. We defined this postoperative swelling of the neck and pharynx in view of the following findings: (1) pharyngeal swelling via direct laryngoscopy, (2) neck swelling from the outside, (3) fiberoptic pharyngoscopy, (4) cuff-leak test [1, 4].
Therapy
Patients received crystalloid solution at 2 ml/kg/h. When urine output was <0.5 ml/kg/h, they additionally received Ringer’s acetate solution at 2 ml/kg/h for 2 h. If this was not effective, 0.2 mg/kg furosemide was administered i.v.. When hemoglobin concentration was <8 g/dl, red cell concentrates were transfused. The nursing staff started vasoactive agent (dopamine) therapy at 5 μg/kg/h when systolic blood pressure was <100 mmHg [7] and adjusted the rate to maintain systolic blood pressure >100 mmHg. The nursing staff also administered atropine when the heart rate was <50 bpm. Patients were monitored by nurses using the Sedation Agitation Scale (SAS) after extubation and until ICU discharge. A diagnosis of agitation was determined by the criterion SAS >0.
Statistical analysis
Results are presented as median (interquartile range [range]) values. Between-group comparisons were made using the Mann–Whitney U test or Fisher’s exact probability test. Within-group comparisons were made using the Wilcoxon test or Friedman test, followed by Wilcoxon test for repeated measures. Statistical analysis was limited to 14 h for repeated measures because of small number of patients. A P value <0.05 was considered statistically significant. For sample size calculation, ≥5% different proportion in adequate sedation level between two sedation agents was clinically important, with a standard deviation of 5% [8], a power minimum of β 0.80, α 0.05, yielding a target population of 16.
Results
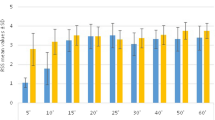
Pathology and surgical procedures are outlined in Table 1. There were no significant differences between the groups in terms of patients’ characteristics, anesthetic and operative durations, blood loss, or blood transfusion during surgery (Table 2). The loading doses of both drugs increased the RSS, and decreased response of movement and minute ventilation (Table 3). Table 4 shows the sedative, responsive, analgesic effects of the two sedatives during the study period, excluding the loading-dose period. Mean maintenance dose of propofol was 1.1 (1.0, 1.3) mg/kg/h and that of dexmedetomidine 0.43 (0.40, 0.46) μg/kg/h. The frequencies of supplementary bolus administration and adjustment of sedative infusion rates, and the number of additional uses of two sedatives were similar between groups. Proportions of adequate sedation, movement of extremities, and pain status were also similar between groups. Heart rate was decreased by dexmedetomidine after loading and up to 14 h (Fig. 1a). Heart rate was decreased by propofol at some time points. Heart rate in group D was less than that in group P after loading and up to 6 h. Both sedatives decreased systolic blood pressure after loading (Fig. 1b) and increased dopamine dose at some time points (Fig. 1c). Although there was no significant difference in systolic blood pressure between groups, dopamine dose in group D was greater than that in group P at 2 h. There were no significant differences between groups in study duration (Table 5). The frequency of atropine use in group D was greater than that in group P. There were no significant differences between groups in the frequency of atropine administration in the loading time (1 in group D vs. 0 in group P). Hemodynamically significant arrhythmia did not occur in either group. There were no significant differences between groups in the frequency of dopamine administration. Although there were no significant differences in the mean amounts of infusion fluid and blood loss (Table 5), mean urinary output in group D was significantly greater than that in group P. There were no significant differences between groups in intubation duration and the number of delayed extubations (Table 6). There were no significant differences in the durations of ICU and hospital stay between groups.
Individual heart rate (bpm) (a), systolic blood pressure (b), and dopamine dose (μg/kg/min) (c) during study periods between propofol and dexmedetomidine. Values are expressed as median (line inside box), with the 25th and 75th percentiles. The capped lines indicate the 10th and 90th percentiles. D dexmedetomidine, P propofol, before before loading, after after loading, # P < 0.05 vs propofol group; $ P < 0.05 vs before value. Statistical analysis was limited to 14 h because of the shortage of numbers
Discussion
Our results showed that there were no significant differences in the proportions of adequate sedation level, movement, and pain status between groups during postoperative sedation after ECSS. Similarly, the frequency of bolus infusion and adjustment in infusion rate, and the frequency of the additional use of the two sedatives, were not different between groups. Dexmedetomidine decreased heart rate and required more frequent atropine administration and a higher dose of dopamine compared with propofol. Postoperative urinary output in group D was greater than that in group P, despite similar amounts of infusion. These findings confirm that both dexmedetomidine and propofol are effective for postoperative sedation after ECSS.
Dexmedetomidine-associated hypotension and bradycardia occur subsequent to sympatholysis resulting from the activation of α2-receptors. Because there is an opposing effect on α2a- and α2b-receptors, dexmedetomidine causes a biphasic change in blood pressure. Dexmedetomidine causes a decrease in mean arterial pressure at a low concentration (<1.9 ng/ml) but an increase at a higher concentration [9]. It is likely that activation of peripheral α2b-receptors at a higher concentration would cause vasoconstriction, thereby offsetting the vasodilation from the activation of α2a-receptors. Critically ill patients receiving a dosage of 0.7 μg/kg/h have shown a peak serum dexmedetomidine concentration of 1.2 ng/ml. This is below the level at which activation of α2b-receptors starts to predominate, which could explain why hypotension is the most common adverse effect [10]. Corbett et al. [11] reported that dexmedetomidine sedation results in a lower heart rate but similar blood pressure compared with propofol sedation after cardiac surgery. Our results show that dexmedetomidine decreased heart rate and thus requires more frequent atropine administration and a higher dopamine dose at 2 h compared with propofol. The discrepancy of the effects on arterial blood pressure might be explained by differences in dosages of dexmedetomidine (0.31 μg/kg/h in Maze et al. vs. 0.43 μg/kg/h in our study).
Postoperative urinary output in group D patients was greater than that in group P patients. The increase in urinary output induced by dexmedetomidine can be attributed to the renal diuretic effects of both dexmedetomidine [12] and dopamine. Dexmedetomidine provides an analgesic effect in a dose-dependent manner [6]. However, in our study, dexmedetomidine was not superior to propofol as an analgesic; this was because pain was relieved at the same fixed dose of buprenorphine in both groups. Sedatives are often used in conjunction with analgesics to provide patient comfort and safety in ICU, especially in mechanically ventilated patients [13]. Adequate analgesia is important, as pain when inappropriately treated can cause tachycardia, immunosuppression, increased catecholamine production, and increased oxygen consumption. We set the continuous buprenorphine infusion at a fixed dosage of 0.3 μg/kg/h, according to a previous report [14]. The median visual analogue scale (VAS) scores in patients undergoing lumbar spinal fusion surgery and receiving continuous buprenorphine at almost the same dose were <30, which is commonly considered satisfactory pain relief. Although buprenorphine might have a sedative effect, the previous report showed that the incidence of sedation by continuous buprenorphine infusion at this dose was comparable to placebo.
Although a previous meta-analysis showed that dexmedetomidine is associated with a significant reduction in the length of ICU stay [15], there was no significant difference in our study. This discrepancy could be due to a difference in study populations, i.e., postoperative vs critically ill patients. ICU stay of some patients without delayed extubation was relatively long. In our previous report [1], two patients were reintubated at 2 or 3 postoperative days due to pharyngeal swelling after ECSS before the prophylactic delayed extubation protocol. At that time, we discharged ECSS patients without delayed extubation from ICU in the 2 POD morning in principle. Moreover, dopamine weaning was necessary for the patient who was given a high dose of dopamine.
Awake craniotomy is performed to facilitate intraoperative functional cortical mapping and neurocognitive testing. The optimal dosage of dexmedetomidine for awake craniotomy is stated to be approximately 0.2 μg/kg/h [16]. It has been reported that sedation with propofol (1.4 ± 0.8 mg/kg/h) diminished the score of a cognitive test, whereas sedation with dexmedetomidine (0.3 ± 0.1 μg/kg/h) maintained the score [17]. Although the higher dose of dexmedetomidine in the study presented here would have caused deeper sedation compared with awake craniotomy, patients were still able to undergo the test of movement of the extremities.
Upper airway compromise after ECSS is a potentially life-threatening condition. Postoperative airway swelling has been reported after carotid endarterectomy, thyroidectomy, upper oropharyngeal procedure, and extensive cervical surgery, including multilevel anterior cervical spine surgery [4] and combined anterior–posterior cervical spine surgery [1]. Some degree of airway obstruction is not uncommon after anterior cervical spine surgery. The causes are tissue swelling of the pharynx in most cases and hematoma in some cases. It usually presents within 6 h, but it can occur later [18]. Therefore, some institutions indicate overnight intubation for patients deemed to be high risk [1, 4, 19, 20]. The main reason for prophylactic intubation overnight is difficulty of emergency reintubation because of postoperative reactive swelling in cases of anterior fusion and anterior–posterior fusion, and postoperative fixed neck in cases of posterior fusion. Significant risk factors include exposure of four or more vertebral bodies that involve C4 or higher and an operative time of >5 h [19]. Epstein et al. [21] reported that the potential risk factors associated with postoperative emergency airway management following cervical spine surgery include the following: obesity (>220 lbs), surgery time >10 h, a second anterior corpectomy with fusion, anterior corpectomy with fusion with C2, >4 U of transfused blood, asthma, advanced age (>65 years), a cerebrospinal fluid fistula, extensive surgery, and severe preoperative neurologic deficit. However, there are few reports on the ideal method of postoperative sedation after ECSS. Although early tracheostomy may not need postoperative sedation, it is neither practical nor beneficial [22].
Our study has the limitation of being conducted in a single center and with a relatively small sample size. This, to a certain extent, is unavoidable, as ECSS is performed in only a limited number of institutions and patients. Admittedly, because the study was not blinded, a theoretical bias by the nurses might have been present. However, as there were more than two dozen nurses blinded to the study purpose and who could be randomly involved in the care of each patient during ICU stay, the likelihood of bias was extremely remote [8]. Although VAS and numeric rating scale are the reliable indicators of pain intensity, sedated patients are often unable to communicate their level of pain [13]. No single tool is universally accepted for use in sedated patients [23]. Therefore, we used more simple scale of pain intensity. However, there are no data and evidence to support its efficacy compared with other pain tools used in sedated patients. The comparatively long hospital stays of some of our patients were caused by surgical-site infection and complications, such as severe rheumatoid arthritis, poliomyelitis, cervical spinal cord injury and tumor, and postoperative rehabilitation. The directions for dexmedetomidine in this study deviated from Japanese pharmaceutical affairs law. If adequate sedation was not achieved at a continuous dexmedetomidine dose, bolus injection of either propofol or midazolam was usually used to provide rescue sedation. Because our intent was to evaluate the sole sedative effect of dexmedetomidine, we permitted use of bolus administration of dexmedetomidine according to a previous study [15]. In a phase IV study, dexmedetomidine was safe in dosages up to 1.4 μg/kg/h [24]. We set the maximum infusion rate at 1 μg/kg/h, according to a previous study [25]. Because conventional sedation scoring systems based on clinical observation may not work well for dexmedetomidine-induced sedation, one study recommended using the combination of bispectral index (BIS) and sedative scale [6]. However, the recommendations in clinical guidelines do not endorse routine use objective measures of sedation, such as BIS in measuring sedation in the ICU [22, 23].
Conclusions
Dexmedetomidine and propofol are both efficacious for sedation after ECSS; however, dexmedetomidine decreased heart rate and required higher doses of dopamine compared with propofol.
References
Terao Y, Matsumoto S, Yamashita K, Takada M, Inadomi C, Fukusaki M, Sumikawa K. Increased incidence of emergency airway management after combined anterior–posterior cervical spine surgery. J Neurosurg Anesthesiol. 2004;16:282–6.
Mashour GA, Stallmer ML, Kheterpal S. Predictors of difficult intubation in patients with cervical spine limitation. J Neurosurg Anesthesiol. 2008;20:110–5.
Tadie JM, Behm E, Lecuyer L, Benhmamed R, Hans S, Brasnu D, Diehl JL, Fagon JY, Guerot E. Post-intubation laryngeal injuries and extubation failure: a fiberoptic endoscopic study. Intensive Care Med. 2010;36:991–8.
Emery SE, Akhavan S, Miller P, Furey CG, Yoo JU, Rowbottom JR, Bohlman HH. Steroids and risk factors for airway compromise in multilevel cervical corpectomy patients. A prospective, randomized, double-blind study. Spine. 2009;34:229–32.
Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109:745–53.
Kasuya Y, Govinda R, Rauch S, Mascha EJ, Sessler DI, Turan A. The correlation between bispectral index and observational sedation scale in volunteers sedated with dexmedetomidine and propofol. Anesth Analg. 2009;109:1811–5.
Backer DD, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Goottignies P, Vincent JL, For the SOAP II Investigators. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med. 2010;362:779–89.
Saito M, Terao Y, Fukusaki M, Makita T, Shibata O, Sumikawa K. Sequential use of midazolam and propofol for long-term sedation in postoperative mechanically ventilated patients. Anesth Analg. 2003;96:834–8.
Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94.
Gerlach AT, Dasta JF, Steinberg S, Martin LC, Cook CH. A new dosing protocol reduces dexmedetomidine-associated hypotension in critically ill surgical patients. J Crit Care. 2009;24:568–74.
Corbett SM, Rebuck JA, Greene CM, Callas PW, Neale BW, Healey MA, Leavitt BJ. Dexmedetomidine does not improve patient satisfaction when compared with propofol during mechanical ventilation. Crit Care Med. 2005;33:940–5.
Maze M, Scarfini C, Cavaliere F. New agents for sedation in the intensive care unit. Crit Care Clin. 2001;17:881–97.
Jacob J, Fraser GL, Coursin DB, Riker RR, Fontaine D, Wittbrodt ET, Chalfin DB, Masica MF, Bjerke HS, Coplin WM, Crippen DW, Fuchs BD, Kelleher RM, Marik PE, Nasraway SA, Murray MJ, Peruzzi WT, Lumb PD. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41.
Kawamata T, Sato Y, Niiyama Y, Omote K, Namiki A. Pain management after lumbar spinal fusion surgery using continuous subcutaneous infusion of buprenorphine. J Anesth. 2005;19:199–203.
Tan JA, Ho KM. Use of dexmedetomidine as a sedative and analgesic agent in critically ill adult patients: a meta-analysis. Intensive Care Med. 2010;36:926–39.
Souter MJ, Rozet I, Ojemann JG, Souter KJ, Holmes MD, Lee L, Lam AM. Dexmedetomidine sedation during awake craniotomy for seizure resection: effects on electrocorticography. J Neurosurg Anesthesiol. 2007;19:38–44.
Mirski MA, Lewin JJ III, LeDroux S, Thompson C, Murakami P, Zink EK, Griswold M. Cognitive improvement during continuous sedation in critically ill, awake and responsive patients: the acute neurological ICU sedation trial (ANIST). Intensive Care Med. 2010;36:1505–13.
Hunningher A, Calder I. Cervical spine surgery. Anaesth Crit Care Pain. 2007;7:81–4.
Sagi HC, Beutler W, Carroll E, Connolly PJ. Airway complication associated with surgery on the anterior cervical spine. Spine. 2002;27:949–53.
Kwon B, Yoo JU, Furey CG, Rowbottom J, Emery SE. Risk factors for delayed extubation after single-stage, multi-level anterior cervical decompression and posterior fusion. J Spinal Disord Tech. 2006;19:389–93.
Epstein NE, Hollingsworth R, Nardi D, Singer J. Can airway complications following multilevel anterior cervical spine surgery be avoided? J Neurosurg. 2001;94(2 suppl):185–8.
Terragni PP, Antonelli M, Fumagalli R. Early vs. late tracheotomy for prevent of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303:1483–9.
Sessler CN, Grap MJ, Ramsay MAE. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12 Suppl 3:S2.
Guinter JR, Kristeller JL. Prolonged infusions of dexmedetomidine in critically ill patients. Am J Health Syst Pharm. 2010;67:1246–53.
Venn RM, Newman PJ, Grounds RM. A phase II study to evaluate the efficacy of dexmedetomidine for sedation in the medical intensive care unit. Intensive Care Med. 2003;29:201–7.
Acknowledgments
This research was supported, in part, by research funds of the Japan Labor Health and Welfare Organization to promote function.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Terao, Y., Ichinomiya, T., Higashijima, U. et al. Comparison between propofol and dexmedetomidine in postoperative sedation after extensive cervical spine surgery. J Anesth 26, 179–186 (2012). https://doi.org/10.1007/s00540-011-1300-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-011-1300-7