Abstract
Goals
The purpose of this study was to evaluate the efficacy of oral cryotherapy to prevent high-dose melphalan-induced stomatitis.
Patients and methods
Eighteen consecutive recipients of allogeneic hematopoietic stem cell transplant conditioned with high-dose melphalan (140 mg/m2) in combination with fludarabine alone or with fludarabine and additional chemotherapy or radiation were enrolled. The severity of stomatitis was graded according to the National Cancer Institute Common Toxicity Criteria. Patients were kept on oral cryotherapy using ice chips and ice-cold water shortly before, during, and for additional 90 min after completion of melphalan administration.
Results
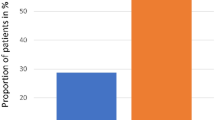
Only two of 18 patients (11.1%) developed grade 2 or 3 stomatitis while six of seven patients in the historical control developed it (85.7%; P=0.001).
Conclusion
These results suggested that oral cryotherapy could effectively prevent stomatitis caused by high-dose melphalan, and we recommend that it should be incorporated into the conditioning regimen with high-dose melphalan.
Similar content being viewed by others
Introduction
High-dose melphalan is frequently used in the conditioning regimen for both autologous and allogeneic hematopoietic stem cell transplantation (HSCT) alone or in combination with other chemotherapeutic agents or total body irradiation (TBI). Recently, high-dose melphalan combined with fludarabine has increasingly been used as a reduced-intensity conditioning regimen in the setting of allogeneic HSCT [2, 4, 6, 7].
Oral mucositis, or stomatitis, is one of the major dose-limiting toxicities of high-dose melphalan and contributes significantly to peritransplant morbidity and sometimes to secondary systemic infections. Oral cryotherapy has proven to be effective to reduce the incidence and severity of stomatitis caused by 5-fluorouracil (5-FU) [1, 8, 10]. Like that of 5-FU, the pharmacokinetics of high-dose melphalan demonstrates a short plasma half-life, which suggest that oral cryotherapy only during the periadministration period could ameliorate the subsequent stomatitis caused by melphalan. In this study, we evaluated the efficacy of oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in recipients of allogeneic HSCT who underwent reduced-intensity conditioning.
Patients and methods
Patients and treatment procedures
Patients with hematologic malignancies who underwent allogeneic HSCT in Keio University Hospital with a reduced-intensity conditioning regimen consisting of fludarabine and high-dose melphalan (140 mg/m2) were consecutively included. Patients who had received the same conditioning regimen for allogeneic HSCT without oral cryotherapy before this study was started served as a historical control.
Basically, patients intravenously received fludarabine at a dose of 25 mg/m2 daily for 5 days (days −6 to −2) and melphalan at a total dose of 70 mg/m2 per day for 15 min for 2 days (days −3 and −2). In the study group, five patients received additional chemotherapy or radiation as conditioning. For the prophylaxis of acute graft-versus-host disease (GVHD), patients received cyclosporine A (CSA) or tacrolimus with or without short-term methotrexate. Administration of lenograstim at a dose of 5 μg/kg was initiated 1 day after HSCT.
Oral cryotherapy
After informed consent had been obtained, patients received oral cryotherapy on each day of melphalan administration. Patients were instructed to put ice chips and ice-cold water in their mouths 15 min before, during, and for additional 90 min after the melphalan infusion. Patients were advised to continuously swirl ice chips around in their mouths and to gargle with and swallow ice-cold water every 10–20 min throughout oral cryotherapy.
Grading of stomatitis
For grading of stomatitis, the National Cancer Institute Common Toxicity Criteria (NCI-CTC) were used. Grading of stomatitis was as follows: Grade 0: none; grade 1: painless ulcers, erythema, and/or mild soreness in the absence of lesions; grade 2: painful erythema, edema, or ulcers, but can swallow; grade 3: painful erythema, edema, or ulcers preventing swallowing or requiring hydration or parenteral (or enteral) nutritional support; grade 4: severe ulceration requiring prophylactic intubation or resulting in documented aspiration pneumonia. The graded degree of stomatitis was evaluated every day from the day of HSCT to day 28, and the maximum grade of each patient was considered as his or her grade.
Statistical analysis
The difference in the incidence of stomatitis was determined by comparing the two groups by using Fisher’s exact test.
Results
Patient characteristics
Eighteen patients were enrolled, and their characteristics are shown in Table 1. In addition to high-dose melphalan and fludarabine, five patients received chemotherapy or radiation such as TBI (400 cGy in three), craniospinal irradiation (240 cGy in one), or high-dose cytarabine (3 g/m2 every 12 h over 2 days in one) as a conditioning regimen.
Incidence and severity of stomatitis
Only two of 18 patients (11.1%) receiving oral cryotherapy developed moderate-to-severe stomatitis (grade 2 or 3 in NCI-CTC) while six of seven (85.7%) subjects in the historical control developed it (P=0.001). Two patients who developed moderate-to-severe stomatitis were conditioned with fludarabine and high-dose melphalan without additional chemotherapy or radiation. No episode of stomatitis of grade 4 was seen among patients in the study group or in the historical control.
Tolerability of oral cryotherapy
Oral cryotherapy was basically well tolerated. Seven patients (39%) complained of chills, and four patients (22%) had nausea during oral cryotherapy. However, none of these events resulted in the discontinuation of oral cryotherapy, except in one case in which oral cryotherapy was discontinued at 60 min after melphalan infusion because of nausea.
Discussion
Stomatitis is one of the prominent features of toxicity of high-dose melphalan (≥140 mg/m2). The incidence and severity of stomatitis depend on the dose of melphalan and probably on the coadministration of other chemotherapeutic agents. Moderate-to-severe stomatitis interferes with nutrition and quality of life and frequently leads to secondary infection in HSCT recipients. Therefore, effective prophylaxis of stomatitis is required as a measure of patient care and, finally, for the improvement of treatment outcome.
Oral cryotherapy for the prevention of stomatitis has already been established in the setting of 5-FU administration [1, 8, 10, 11]. Oral cryotherapy during the periadministration period is only effective for chemotherapeutic agents with short plasma life time. Because of melphalan’s short plasma half-life, two groups evaluated the efficacy of oral cryotherapy to eliminate stomatitis caused by high-dose melphalan in an autologous HSCT setting [3, 9]. Dumonet et al. first reported the efficacy of oral cryotherapy in recipients of autologous HSCT conditioned with various regimens, including high-dose melphalan alone or in combination with total TBI, and BEAM chemotherapy [3]. In their study, oral cryotherapy eliminated the development of severe stomatitis in patients receiving high-dose melphalan alone while it was less effective in patients receiving BEAM or combination therapy with TBI. Meloni et al. also reported the efficacy of oral cryotherapy in preventing stomatitis by using ice pops to ameliorate the uncomfortable symptoms due to oral cryotherapy [9].
With the recent emergence of reduced-intensity regimens, high-dose melphalan has become recognized as one of the important agents, particularly in combination with fludarabine, in the conditioning regimens for allogeneic HSCT. However, moderate-to-severe stomatitis was observed in 60–80% of patients undergoing this regimen [2, 6, 7]. In this study, the incidence of moderate-to-severe stomatitis in patients receiving high-dose melphalan-based reduced-intensity conditioning regimens in conjunction with oral cryotherapy was only 11.1%. In spite of the small number of patients and their heterogeneity, the incidence of stomatitis was significantly lower than that of the historical control in which the incidence (85.7%) was similar to that reported in previous studies [2, 6, 7]. Furthermore, five of the patients received additional chemotherapy or radiation which could be associated with stomatitis. However, these additional therapies were only given to the patients receiving oral cryotherapy, and the reduction in the incidence of stomatitis was successfully achieved even in five patients receiving additional chemotherapy or radiation.
The mechanisms of the efficacy of oral cryotherapy in eliminating stomatitis have not been elucidated, and hypothesis includes local vasoconstriction causing less exposure to melphalan, temperature-dependent reduction of melphalan’s cytotoxicity, reduced production of chemical mediators, and mucous membrane stability.
In this study, patients were asked to continue oral cryotherapy for 90 min after completion of melphalan infusion. The time course of this procedure was determined according to melphalan’s plasma half-life after high-dose administration. The pharmacokinetics of high-dose melphalan demonstrated a biphasic plasma half-life, a distribution phase t1/2, 6–16 min, and an elimination phase t1/2, 40–83 min (Gouyette et al. [5] and an internal report of GlaxoSmithKline Inc.). It is strongly suggested that its distribution phase, because the concentration is higher during the distribution phase, plays a more crucial role in causing stomatitis than does the elimination phase. Therefore, the optimal time for oral cryotherapy could be shortened which may lead to elimination of unpleasant symptoms (e.g., chills or nausea) associated with this procedure.
These findings suggested that oral cryotherapy could be an effective and reasonable therapy for the prevention of development of stomatitis caused by high-dose melphalan in an allogeneic HSCT setting. Future randomized trials are required to further evaluate its efficacy and also to determine the appropriate time course.
References
Cascinu S, Fedeli A, Fedeli SL, Catalano G (1994) Oral cooling (cryotherapy), an effective treatment for the prevention of 5-fluorouracil-induced stomatitis. Oral Oncol, Eur J Cancer 30B:234–236
Devine SM, Sanborn R, Jessoop E, Stock W, Huml M, Peace D, Wickrema A, Yassine M, Amin K. Thomason D, Chen Y-H, Devine H, Maningo M, van Besien K (2001) Fludarabine and melphalan-based conditioning for patients with advanced hematological malignancies relapsing after a previous hematopoietic stem cell transplant. Bone Marrow Transplant 28:557–562
Dumonet C, Sonnet A, Bastion Y, Salles G, Espinouse D, Coiffier B (1994) Prevention of high dose L-PAM-induced mucositis by cryotherapy. Bone Marrow Transplant 3:492-494
Giralt S, Aleman A, Anagnostopoulos A, Weber D, Khouri I, Anderlini P, Molldrem J, Ueno NT, Donato M, Korbling M, Gajewski J, Alexanian R, Champlin R (2002) Fludarabine/melphalan conditioning for allogeneic transplantation in patients with multiple myeloma. Bone Marrow Transplant 30:367–373
Gouyette A, Hartman O, Pico J-L (1986) Pharmacokinetics of high-dose melphalan in children and adults. Cancer Chemother Pharmacol 16:184–189
Kroger N, Schwerdrfeger R, Kiehl M, Sayer HG, Renges H, Zabelina T, Fehse B, Togel F, Wittkowsky G, Kuse R, Zander AR (2002) Autologous stem cell transplantation followed by a dose-reduced allograft induces high complete remission rate in multiple myeloma. Blood 100:755–760
Kroger N, Sayer HG, Schwerdtfeger R, Kiehl M, Nagler A, Renges H, Zabelina T, Fehse B, Ayuk F, Wittkowsky G, Schmitz N, Zander AR (2002) Unrelated stem cell transplantation in multiple myeloma after a reduced-intensity conditioning with pretransplantation antithymocyte globulin is highly effective with low transplant-related mortality. Blood 100:3919–3924
Mahood DJ, Dose AM, Loprinzi CL, Veeder MH, Athmann LM, Therneau TM, Sorensen JM, Gainey DK, Mailliard JA, Gusa NL, Finck Gk, Johnson C, Goldberg RM (1991) Inhibition of fluorouracil-induced stomatitis by oral cryotherapy. J Clin Oncol 9:449–452
Meloni G, Capria S, Proia A, Trisolini SM, Mandelli F (1996) Ice pops to prevent melphalan-induced stomatitis. Lancet 347:1691–1692
Rocke LK, Loprinzi CL, Lee JK, Kunselman SJ, Iverson RK, Finck G, Lifsey D, Glaw KC, Stevens BA, Hatfield AK, Vaught NL, Bartel J, Pierson N (1993) A randomized clinical trial of two different durations of oral cryotherapy for prevention of fluorouracil-related stomatitis. Cancer 72:2234–2238
Rubenstein EB, Peterson DE, Schubert M, Keefe D, McGuire D, Epstein J, Elting LS, Fox PC, Cooksley C, Sonis ST (2004) Clinical practice guidelines for the prevention and treatment of cancer therapy-induced oral and gastrointestinal mucositis. Cancer 100 (Suppl 9):2026–2046
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aisa, Y., Mori, T., Kudo, M. et al. Oral cryotherapy for the prevention of high-dose melphalan-induced stomatitis in allogeneic hematopoietic stem cell transplant recipients. Support Care Cancer 13, 266–269 (2005). https://doi.org/10.1007/s00520-004-0726-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-004-0726-y