Abstract
Background
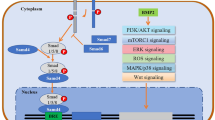
Transforming growth factor-β (TGF-β) is known to promote tumor proliferation, migration, invasion, and metastasis. Bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily. Several BMPs (BMP2 and BMP7) can enhance the invasion and bone metastasis of breast cancer cells. The function of BMP9, the latest discovered and most powerful osteogenetic factor, in breast cancer has not been fully elucidated.
Methods
BMP9 expression in twenty-three breast cancer patients and three breast cancer cell line types was detected by reverse transcriptase polymerase chain reaction. Changes in proliferation, apoptosis, invasion, and migration in the recombinant MDA-MB-231/BMP9 cells were detected using various assays. The assays were MTT, flow cytometry, colony forming, cell wounding, and transwell invasion. Proliferating cell nuclear antigen and terminal deoxynucleotidy transferase biotin-dUTP nick end labeling staining methods were conducted to detect whether BMP9 affected proliferation and apoptosis in xenogenic mouse models.
Results
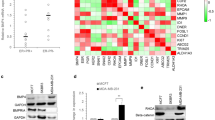
Twenty-one of the twenty-three breast cancer patients had amplified BMP9 mRNA transcripts in adjacent non-tumor tissues, although BMP9 was observed in the breast cancer tissue of two patients, its expression was higher in the adjacent non-tumor tissues. BMP9 overexpression inhibited the proliferation, migration, and invasion, as well as induced the apoptosis of the breast cancer cell line MDA-MB-231 in vitro. BMP9 also inhibited tumor growth and induced apoptosis significantly in the xenogenic mouse models.
Conclusions
Decreased BMP9 expression is associated with the elevated proliferation and migration of human breast cancer. BMP9 can inhibit the growth, invasion, and migration of breast cancer cells in vitro and in vivo. BMP9 is a putative tumor suppressor in breast cancer.





Similar content being viewed by others
References
Alarmo EL, Korhonen T (2008) Bone morphogenetic protein 7 expression associates with bone metastasis in breast carcinomas. Ann Oncol 19:308–314
Alarmo EL, Parssinen J, Ketolainen JM et al (2009) BMP7 influences proliferation, migration, and invasion of breast cancer cells. Cancer Lett 275:35–43
Blanca H, van Maarten D, ten Peter D et al (2009) Autocrine bone morphogenetic protein-9 signals through activin receptor-like Kinase-2/Smad1/Smad4 to promote ovarian cancer cell proliferation. Cancer Res 69:9254–9262
Buijs JT, Henriquez NV, van Overveld PGM et al (2007) Bone morphogenetic protein 7 in the development and treatment of bone metastases from breast cancer. Cancer Res 67:8742–8751
Celeste AJ, Song JJ, Cox K et al (1994) Bone morphogenetic protein-9, a new member of the TGF-β superfamily. J Bone Miner Res Suppl1:136
Cheng H, Jiang W, Phillips FM et al (2003) Osteogenic Activity of the 14 Types of Human Bone Morphogenetic Proteins (BMPs). Journal of Bone and Joint Surgery 85:1544–1552
Du J, Yang S, Wang Z et al (2008) Bone morphogenetic protein 6 inhibit stress-induced breast cancer cells apoptosis via both Smad and p38 pathways. J Cell Biochem 103:1584–1597
Feng XH, Derynck R (2005) Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Bio 21:659–693
Hatakeyama S, Ohara-Nemoto Y, Kyakumoto S et al (1993) Expression of bone morphoenetic protein in human adencarcinoma cell line. Biochem Biophys Res Commun 190:695–701
Katsuno Y, Hanyu A, Kanda H et al (2008) Bone morphogenetic protein signaling enhances invasion and bone metastasis of breast cancer cells through Smad pathway. Oncogene 27:6322–6333
Kim IY, Lee DH, Ahn HJ et al (2000) Expression of bone morphogenetic protein receptors type IA,- IB and–II correlates with tumor grade in human prostate cancer tissues. Cancer Res 60:2840–2844
Langenfeld EM, Caivano SE, Abou-Nukta F et al (2003) The mature bone morphogenetic protein-2 is aberrantly expressed in non-small cell lung carcinomas and stimulates tumor growth of A549 cells. Carcinogenesis 24:1445–1454
Laurent D, Jean-Jacques F, Sabine B (2009) Emerging role of bone morphogenetic proteins in angiogenesis. Cytokine & Growth Factor Reviews 20:203–212
Li JZ, Li H, Sasaki T et al (2003) Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther 10:1735–1743
Lin YE, Bokobza SM, Jiang WG (2009) Bone morphogenetic proteins in development and progression of breast cancer and therapeutic potential. International Journal of Molecular Medicine 24:591–597
Lowery JW, Caestecker MP (2010) BMP signaling in vascular development and disease. Cytokine &Growth factor reviews 21:287–298
Majumdar MK, Wang E, Morris EA et al (2001) BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol 189:275–284
Ploemacher RE, Engels LJ, Mayer AE et al (1999) Bone mophogenetic protein 9 is a potent synergistic factor for murine hemopoietic progenitor cell generation and colony formation in serum-free cultures. Leukemia 13:428–437
Singh A, Morris RJ (2010) The Yin and Yang of bone morphogenetic proteins in cancer. Cytokine &Growth factor reviews 21:299–313
Song JJ, Celeste AJ, Kong FM et al (1995) Bone mophogenetic protein -9 binds to liver cells and stimulates proliferation. Endocrinology 136:4293–4297
Wagner DO, Sieber C, Bhushan R et al (2010) BMPs: From Bone to Body Morphogenetic Proteins. Science Signaling 3:1–6
Ye L, Howard K, Jiang WG (2008) Bone Morphogenetic Protein-9 Induces Apoptosis in Prostate Cancer Cells, the Role of Prostate Apoptosis Response-4. Mol Cancer Res 10:1594–1606
Acknowledgments
The authors would like to thank Dr. LIU Shengchun for the human breast cancer tissue samples. The present study was supported by the National Natural Science Foundation of China (Grant No. 30800658), the National Natural Science Foundation of China (Grant No. 81172017) and the Natural Science Foundation Project of Chongqing Science and Technology Commission (Grant No. 2009BB5060).
Conflict of Interest
I declared no Conflict of Interest Statement.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ke Wang and Honglei Feng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Wang, K., Feng, H., Ren, W. et al. BMP9 inhibits the proliferation and invasiveness of breast cancer cells MDA-MB-231. J Cancer Res Clin Oncol 137, 1687–1696 (2011). https://doi.org/10.1007/s00432-011-1047-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-011-1047-4