Abstract
Less invasive surfactant administration or minimally invasive surfactant therapy (LISA/MIST) has been proposed for the administration of surfactant in preterm infants without intubation. The aim of our survey was to assess the rate of utilization, premedication as well as technique and equipment used for LISA/MIST. Furthermore, attitudes and experiences in regard to indications, side effects, and efficacy should be assessed. An online-based survey was sent to 324 neonatologists from different centers within 37 European countries between December 2015 and March 2016. Of those 165 who responded (response rate 51%), 86 (52%) were using LISA/MIST. It is regarded the standard procedure for surfactant administration by 41%, with a wide variation in personal views on patient selection in terms of indication, appropriate gestational and postnatal age. Policies concerning premedication, devices, and technique of LISA/MIST differed widely. Side effects like surfactant reflux, bradycardia, and hypoxia were observed by 77% of neonatologists. Of neonatologists inexperienced in LISA/MIST, 89% would consider utilizing it in the future. Perceived efficacy of LISA/MIST was high (52%) to medium (33%).
Conclusion: The use of LISA/MIST within Europe is widespread. There is a wide variation concerning all aspects of LISA in daily clinical routine and different views on when and how LISA should be performed.
What is Known: • Noninvasive surfactant administration has been the subject of randomized controlled trials and has found its way into clinical routine. |
What is New: • Noninvasive surfactant administration techniques are widely applied in European neonatal units. • There is a wide variety of equipment used and techniques applied for less invasive surfactant delivery as well as different views on the indications and perceived efficacy of this intervention. |
Similar content being viewed by others
Introduction
Surfactant therapy decreases mortality and morbidity in premature infants [20, 22, 23]. In the wake of using noninvasive respiratory support for premature infants in the delivery room, the question of how to replace exogenous surfactant emerged and methods other than intubation were investigated: an approach of tracheal intubation, surfactant administration, and rapid extubation (INSURE) is widely adopted [25]. However, to fully avoid mechanical ventilation, brief tracheal catheterization with a thin catheter for surfactant administration during spontaneous breathing was proposed in 1992 [24]. This technique comprises the use of a laryngoscope and optionally a Magill forceps for placing the orally or nasally inserted catheter beyond the vocal cords. Surfactant is slowly administered into the trachea while the infant is breathing spontaneously on noninvasive respiratory support. This method of surfactant therapy has been referred to as “Less Invasive Surfactant Administration” (LISA), “Minimally Invasive Surfactant Therapy” (MIST), or “Surfactant Without Intubation” (SWI). This technique was revived and refined by Kribs et al. in 2001 [15, 17]. For the purpose of this article, the acronym LISA will incorporate all methods of surfactant application with a thin endotracheal catheter during spontaneous breathing.
Feasibility, safety, and efficacy of LISA have been subjects of prospective randomized controlled and observational trials with similar short-term outcomes compared to the standard approach. Within these trials, several methods to perform LISA have been described involving different modalities and equipment [1, 6, 7, 10–14, 16].
The aim of our survey was to improve the knowledge about the current use of LISA within Europe: Perceptions of indication and feasibility in regard to gestational age, policy of premedication, technique, and equipment as well as experience of side effects and efficacy should be depicted in this survey.
Materials and methods
A structured and stratified questionnaire was sent to 324 neonatologists of different neonatal intensive care units (NICU) within 37 European countries between December 2015 and March 2016 using an online survey tool (SurveyMonkey, Portland, OR).
Level of neonatal care, unit size, and general use of LISA were inquired. Clinicians using LISA were asked about unit policy, technique, and equipment. Furthermore, we evaluated personal views in regard to patient selection, experiences of side effects, and perception of efficacy. For neonatologists who had used LISA but have ceased using it, the survey proceeded with questions about their reasons for discontinuation and their current alternative strategies for surfactant treatment. Physicians, who have never used LISA, were asked about their preferred method. Survey respondents were contacted by email or phone to clarify incomplete or inconsistent data if necessary.
Data analysis was descriptive. Categorical variables are presented in absolute numbers and percentages, and quantitative data are reported as median and interquartile range. Data analysis was performed using SigmaPlot (ver. 11, Systat Software, San Jose, CA).
Results
Neonatologists from 324 different units were contacted, and 165 (51%) completed the survey (Table 1). Three opted out to participate, and 155 (48%) did not respond after additional reminders. Contact data were incorrect for four units. Seven participants were contacted for clarification of data. Of the participating NICUs 150 (91%) provided level III and 15 (9%) level II of neonatal care (definition according to the American Academy of Pediatrics). The median number of inborn very low birth weight infants per year in participating units was 80 (IQR 50–120).
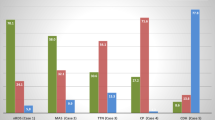
By the time of the survey, LISA was used by 86 of the responding neonatologists (52%) (Fig. 1). In 41 of the units (48%), LISA is used regularly and in 31 (36%) occasionally (Table 2). Forty-one percent of neonatologists applying LISA consider it the standard procedure for surfactant administration, and 53/86 (62%) units follow a standardized written protocol on LISA within their department.
Thirty-eight percent of neonatologists would apply LISA irrespective of the infant’s gestational age and 42% irrespective of the infant’s postnatal age. Forty-five percent of the respondents would perform LISA in infants below 24 weeks of gestational age (Fig. 2a, b).
There was a wide variation in personal views concerning the indications for LISA. Forty-nine percent of respondents would not consider LISA for prophylaxis of respiratory distress syndrome (RDS), 46% would not use LISA for treatment of severe RDS, and 25% of respondents would not treat mild RDS with LISA.
Fifty-two percent of neonatologists are not using any form of sedation for LISA. The remainder uses atropine (29%), opioids (23%), ketamine (9%), propofol (8%), caffeine (6%), benzodiazepines (5%), and others drugs (6%) in different combinations of up to four drugs (Table 2).
Regular feeding tubes, vascular catheters, suction catheters, Foley catheters, large bore arterial catheters, and custom made devices are used for delivering surfactant (Table 2). The catheters are shortened by 24% of the respondents. Catheter sizes ranged from 2.5 to 5 French.
The majority of neonatologists use a Magill forceps to introduce the catheter into the trachea (56/86). Eighteen percent of those (10/56) keep the catheter in place with the forceps while administering surfactant. The oral route is favored over the nasal route (65 vs 26%). Only 17% of respondents instill surfactant under continuous visualization using a laryngoscope. Forty-six percent of respondents give surfactant in less than 1 min. In most units, the dose of surfactant given by LISA does not differ from the dose of surfactant given invasively. Four of the respondents would increase, and one would decrease the dose. If a second dose of surfactant is indicated, 56% of respondents would repeat LISA, whereas the remainder would intubate the infant or perform INSURE.
Side effects of LISA were experienced by 77% of neonatologists. Tracheal surfactant reflux (69%), bradycardia (41%), and hypoxia (34%) among others were observed. Perceived efficacy of LISA among participating neonatologists was high (Table 3).
LISA was never applied in 68 NICUs (41%): Reasons for not using LISA were lacking experience (22/68), inconclusive evidence for the use of LISA, and missing consensus on when and how to perform LISA (23/68). However, 89% of neonatologists inexperienced in LISA would consider using LISA given the opportunity to become familiar with this technique.
In 10 units (6%), LISA was applied in the past but discontinued due to low efficacy (n = 4), inconclusive evidence on technique how to perform LISA (n = 6), or an unacceptable rate of adverse events. INSURE (38/78) or intubation (35/78) was used for surfactant therapy instead.
Discussion
With the call for a more noninvasive approach in the care for preterm infants, LISA has been promoted as an alternative way for surfactant administration.
To date, more than half of the participating European units incorporate LISA into their management. We observed a marked variation of its use between countries. However, the number of replies from some countries is too low to draw a representative picture for the overall use of LISA in Europe. Additional data are available only for Poland where a national survey revealed less than 1% LISA use in 2015 [3].
Interestingly, the majority of neonatologists consider LISA even in extremely immature infants. Observational data from the German Neonatal Network confirm the frequent use of LISA even in the most immature neonates [10]. This may be in line with results of a recent study in infants 23–26 weeks gestational age, where increased survival without major complications was seen in the group of infants treated with LISA. However, subgroup analysis from this study revealed the best benefit in the more mature infants of 25 and 26 weeks [16].
The fact, that almost half of the neonatologists would not use LISA to treat severe RDS and that a LISA treatment failure would be followed by other methods to deliver surfactant may show that LISA is regarded a safe first-line attempt but may be reserved for the less sick infants. Invasive ventilation may represent the usual approach for infants presenting with severe RDS.
For intubation of neonates, international guidelines recommend sedation in nonemergency situations [2, 5]. Although this topic is debated and recommendations are not consistently applied within Europe, many neonatologists would recommend sedation or muscle relaxation for neonatal intubation [4, 21]. In contrast, 52% of neonatologists would not use any premedication for performing LISA. We speculate that compared to invasive intubation some neonatologists perceive LISA being less traumatic or maintenance of spontaneous breathing being superior to analgesia or sedation. The remaining neonatologists use a wide variation of sedative drugs in various combinations for premedication. Substances to alleviate pain as lidocaine spray, oral sucrose, opioids, and ketamine may be indicators that these neonatologists consider LISA to be painful or unpleasant. The use of muscle relaxant and sedatives may accelerate tracheal placement of the catheter in vigorous preterm infants or may prevent surfactant reflux. However, the use of these regimens may have adverse effects on respiratory drive, may induce protracted arterial hypotension or may lead to tracheal intubation more frequently, as compared to non-sedated infants [9, 26]. According to our survey, no consensus for premedication is evident among neonatologists performing LISA nor is a uniform approach apparent from the published literature. However, the analysis of perceived efficacy of LISA and use of premedication showed no association in our survey.
Purpose-made devices for LISA are currently under development (personal communication). To date, the majority of respondents are using feeding tubes and vascular catheters for LISA. However, the presence of two separate perforations at the tip of the feeding tubes may lead either to a surfactant reflux via the upper perforation or unilateral surfactant deposition when the catheter is inserted deeply. Shortening of the catheter is performed by 24% of respondents. This may pose the risk of mucosal injury and bleeding by creating sharp edges.
Inadvertent loss of surfactant phospholipids using thin catheters was reported to be significantly higher than using larger endotracheal tubes [8]. Nevertheless, most neonatologists would not adjust the surfactant dose. Few replies suggested increasing the dose of surfactant for LISA; unfortunately, their rationale for increasing the dose was not substantiated by our survey.
Holding the catheter in place with a Magill forceps during surfactant administration may prevent dislocation of the catheter in a vigorous infant but may cause additional oral leak and stress. Similarly, administration of surfactant under continuous visualization allows better monitoring of the surfactant deposition but increases time of laryngoscopy and thus causes potential discomfort. Furthermore, it creates a large oral leakage. Subsequently, continuous positive airway pressure, a basic factor within the concept of LISA, may become ineffective. Only a minority of users preferred this modification.
Relevant side effects of LISA have been observed: According to our survey, respondents have frequently experienced the need for tracheal intubation following LISA. The impact of LISA is difficult to unravel since also progressive respiratory distress, increasing fatigue, and apnea may be accountable for respiratory failure. Hypoxia or bradycardia can be observed both during LISA and during surfactant administration in intubated infants. Airway pressure and supplemental oxygen may be increased, but this may be less effective during noninvasive ventilation compared to respirator adjustments in mechanically ventilated infants. In this scenario, noninvasive intermittent positive pressure ventilation was shown to reduce the need for intubation [19]. We cannot specify if the side effects reported were caused by the equipment in use or by the surfactant administration itself.
Tracheal reflux of surfactant is the most common adverse event observed. It may impede sufficient surfactant deposition in the lung. While some surfactant reflux can be observed when using a tracheal tube for surfactant delivery, the fraction of surfactant lost may be higher when applying LISA. In our experience, the failure to improve lung function, observation of tracheal surfactant reflux, or recovery of a substantial amount of surfactant in the gastric aspirate may indicate a substantial misrouting of the surfactant. Nevertheless, in an animal model, efficacy of surfactant therapy was similar during noninvasive and invasive ventilation despite a somewhat lower surfactant deposition [18]. Of those performing LISA, most would consider a second attempt before intubation.
A considerable number of respondents observed unilateral surfactant deposition. This may result in unilateral or localized hyperinflation, lung overdistension, pulmonary interstitial emphysema, and air leaks. However, such sequelae are not reported in our survey and such concerns are not substantiated by the current literature [7, 10, 11, 13, 15, 16].
There are limitations concerning our survey. The response rate was only 51%, and the number of responses varied between countries. Most responses came from level III units with high patient loads. Responses were not substantiated by solid data control. Nevertheless, we presented a broad overview on the application of this technique outside of clinical trials within Europe.
Conclusions
In conclusion, noninvasive surfactant administration techniques are widely applied in European neonatal units. There is a wide variety of equipment and techniques in use for less invasive surfactant administration. There are widely differing views on the indications and perceived efficacy among neonatologists performing LISA.
Abbreviations
- INSURE:
-
Intubation, surfactant, extubation
- LISA:
-
Less invasive surfactant administration
- MIST:
-
Minimally invasive surfactant administration
- NICU:
-
Neonatal intensive care unit
- RDS:
-
Respiratory distress syndrome
- SWI:
-
Surfactant without intubation
References
Ali E, Abdel WM, Alsalami Z, Abouseif H, Gottschalk T, Rabbani R, Zarychanski R, Abou-Setta AM (2016) New modalities to deliver surfactant in premature infants: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 29:3519–3524
Anand KJ (2001) International Evidence-Based Group for Neonatal Pain: consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 155:173–180
Borszewska-Kornacka MK, Kostuch M, Korbal P, Krajewski P, Polish RDS Study Group (2015) Strategies of using surfactant results of the first polish national survey of daily practice. Dev Period Med 19:271–276
Chaudhary R, Chonat S, Gowda H, Clarke P, Curley A (2009) Use of premedication for intubation in tertiary neonatal units in the United Kingdom. Paediatr Anaesth 19:653–658
Committee on Fetus and Newborn and Section on Anesthesiology and Pain Medicine (2016) Prevention and management of procedural pain in the neonate: an update. Pediatrics 137:e20154271
Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG (2011) Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 96:F243–F248
Dargaville PA, Aiyappan A, De Paoli AG, Kuschel CA, Kamlin CO, Carlin JB, Davis PG (2013) Minimally-invasive surfactant therapy in preterm infants on continuous positive airway pressure. Arch Dis Child Fetal Neonatal Ed 98:F122–F126
De Luca D, Minucci A, Gentile L, Capoluongo ED (2014) Surfactant inadvertent loss using feeding catheters or endotracheal tubes. Am J Perinatol 31:209–212
Dekker J, Lopriore E, Rijken M, Rijntjes-Jacobs E, Smits-Wintjens V, Te Pas A (2016) Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 109:308–313
Göpel W, Kribs A, Hartel C, Avenarius S, Teig N, Groneck P, Olbertz D, Roll C, Vochem M, Weller U, von der WA, Wieg C, Wintgens J, Preuss M, Ziegler A, Roth B, Herting E (2015) Less invasive surfactant administration is associated with improved pulmonary outcomes in spontaneously breathing preterm infants. Acta Paediatr 104:241–246
Göpel W, Kribs A, Ziegler A, Laux R, Hoehn T, Wieg C, Siegel J, Avenarius S, von der WA, Vochem M, Groneck P, Weller U, Moller J, Hartel C, Haller S, Roth B, Herting E (2011) Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet 378:1627–1634
Isayama T, Iwami H, McDonald S, Beyene J (2016) Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: a systematic review and meta-analysis. JAMA 316:611–624
Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U (2013) Surfactant administration via thin catheter during spontaneous breathing: randomized controlled trial. Pediatrics 131:e502–e509
Kribs A, Hartel C, Kattner E, Vochem M, Kuster H, Moller J, Muller D, Segerer H, Wieg C, Gebauer C, Nikischin W, Wense A, Herting E, Roth B, Gopel W (2010) Surfactant without intubation in preterm infants with respiratory distress: first multi-center data. Klin Padiatr 222:13–17
Kribs A, Pillekamp F, Hunseler C, Vierzig A, Roth B (2007) Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr Anaesth 17:364–369
Kribs A, Roll C, Gopel W, Wieg C, Groneck P, Laux R, Teig N, Hoehn T, Bohm W, Welzing L, Vochem M, Hoppenz M, Buhrer C, Mehler K, Stutzer H, Franklin J, Stohr A, Herting E, Roth B (2015) Nonintubated surfactant application vs conventional therapy in extremely preterm infants: a randomized clinical trial. JAMA Pediatr 169:723–730
Kribs A, Vierzig A, Hunseler C, Eifinger F, Welzing L, Stutzer H, Roth B (2008) Early surfactant in spontaneously breathing with nCPAP in ELBW infants--a single centre four year experience. Acta Paediatr 97:293–298
Niemarkt HJ, Kuypers E, Jellema R, Ophelders D, Hutten M, Nikiforou M, Kribs A, Kramer BW (2014) Effects of less-invasive surfactant administration on oxygenation, pulmonary surfactant distribution, and lung compliance in spontaneously breathing preterm lambs. Pediatr Res 76:166–170
Oncel MY, Arayici S, Uras N, Alyamac-Dizdar E, Sari FN, Karahan S, Canpolat FE, Oguz SS, Dilmen U (2016) Nasal continuous positive airway pressure versus nasal intermittent positive-pressure ventilation within the minimally invasive surfactant therapy approach in preterm infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 101:F323–F328
Rojas-Reyes MX, Morley CJ, Soll R (2012) Prophylactic versus selective use of surfactant in preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev. doi:10.1002/14651858.CD000510.pub2
Simon L, Trifa M, Mokhtari M, Hamza J, Treluyer JM (2004) Premedication for tracheal intubation: a prospective survey in 75 neonatal and pediatric intensive care units. Crit Care Med 32:565–568
Speer CP, Sweet DG, Halliday HL (2013) Surfactant therapy: past, present and future. Early Hum Dev 89(Suppl 1):S22–S24
Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GH, Halliday HL (2016) European consensus guidelines on the management of respiratory distress syndrome - 2016 update. Neonatology 111:107–125
Verder H, Agertoft L, Albertsen P, Christensen NC, Curstedt T, Ebbesen F, Greisen G, Hobolth N, Holm V, Jacobsen T (1992) Surfactant treatment of newborn infants with respiratory distress syndrome primarily treated with nasal continuous positive air pressure. A pilot study. Ugeskr Laeger 154:2136–2139
Verder H, Robertson B, Greisen G, Ebbesen F, Albertsen P, Lundstrom K, Jacobsen T (1994) Surfactant therapy and nasal continuous positive airway pressure for newborns with respiratory distress syndrome. Danish-Swedish Multicenter Study Group. N Engl J Med 331:1051–1055
Welzing L, Kribs A, Eifinger F, Huenseler C, Oberthuer A, Roth B (2010) Propofol as an induction agent for endotracheal intubation can cause significant arterial hypotension in preterm neonates. Paediatr Anaesth 20:605–611
Acknowledgements
The authors would like to thank Elena Neumann, Ruth Creutzfeldt, Dimitra Stavropoulou for linguistic advice, and Prof. Ewa Helwich, Neonatal Intensive Care Unit, Institute of Mother and Child, Warsaw, Poland, for providing additional information on the use of LISA in Poland, and all participants of this survey—Austria: Universitätsklinik für Kinder-und Jugendheilkunde, Salzburg: M. Wald; Innsbruck Medical University: U. Kiechl-Kohlendorfer; Medical University Graz: B. Urlesberger. Belgium: Centre Hospitalier Régional de la Citadelle, Liège: V. Rigo; C.H.U. Saint-Pierre, Bruxelles: M. Tackoen; Antwerp University Hospital: P. van Reempts; Universitair Ziekenhuis Bruxelles: F. Cools; Ziekenhuis Oost-Limburg, Genk: C. Theyskens; CHC-Site St Vincent, Liege-Rocourt: P. Maton; Ghent University Hospital: K. Smets. Bulgaria: University Hospital “St. Anna”, Varna: M. Marinova-Achkar; University Hospital “Prof. St. Kirkovich”, Stara Zagora: H. Mumdzhiev; UMHAT “St George” Neonatology Clinic, Plovdiv: M. Krasteva. Bosnia and Herzegovina: University Clinical Center Sarajevo: S. Heljic. Cyprus: Hospital Archbishop Makarios III, Nicosia: P. Yiallouros. Croatia: University Hospital Osijek: V. Milas; University Hospital Center Rijeka: I. Čače; University Hospital Center “Sisters of Mercy”, Zagreb: R. Ribičić. Czech Republic: General University Hospital Prague: R. Plavka; The Institute for the Care of Mother and Child, Prague-Podoli: Z. Straňák. Denmark: Copenhagen University Hospital, Rigshospitalet: G. Greisen; Hans Christian Andersen Children’s Hospital: G. Zachariassen. Estonia: Tartu University Hospital: H. Varendi. Finland: Tampere University Hospital: O. Tammela; Oulu University Hospital: M. Hallmann/T. Saarela. France: Centre Hospitalier Universitaire de Limoges: A. Bedu; Centre Hospitalier Régional Universitaire de Strasbourg: D. Astruc; Centre Hospitalier Régional Universitaire de Tours: E. Saliba; Centre Hospitalier Universitaire de Caen: B. Guillois/C. Alexandre; Hôpital de la Croix-Rousse, Lyon: J. Picaud; Groupe Hospitalier Cochin-Broca-Hôtel Dieu, Paris: P. Jarreau; Centre Hospitalier Universitaire Montpellier: C. Milesi; University Children’s Hospital, Bordeaux: O. Brissaud; CHU Estaing: M. Lang; CHRU Rennes: C. Lallemant; CHU Amiens-Picardie: P. Tourneux; Centre Hospitalier Universitaire de Nice: M. Saint-Faust; CHU de Nîmes: M. Di Maio; Groupe Hospitalier du Havre: J. Mourdie; Centre Hospitalier de la Côte Basque: P. Jouvencel; Centre Hospitalier Félix Guyon, Réunion: D. Ramful; North University Hospital, Marseille: C. Grosse. Germany: Universitätsklinikum Würzburg: E. Frieauff; Universitätsklinikum Schleswig-Holstein, Campus Lübeck: W. Göpel/C. Härtel; Universitätsklinikum Tübingen: A. Franz; Universitätsklinikum Ulm: R. Hopfner; Universitätsklinikum des Saarlandes, Homburg: S. Meyer; Schwarzwald-Baar Klinikum Villingen: M. Henschen; Hegau-Bodensee-Klinikum Singen: A. Trotter; Universitätsklinikum Heidelberg: D. Frommhold; Universitätsklinikum Dresden: D. Konstantelos; Universitätsklinikum Leipzig: C. Gebauer; Olgahospital Stuttgart: M. Vochem; Universitätsmedizin Göttingen: H. Küster; Universitätsmedizin Mannheim: T. Schaible; LMU, Klinikum der Universität München: A. Flemmer; Universitätsklinikum Bonn: A. Müller; Vestische Kinder- und Jugendklinik Datteln: C. Roll; Universitätsklinikum Düsseldorf: T. Höhn; Universitätsklinikum Essen: U. Felderhoff-Müser; Universitätsklinikum Frankfurt am Main: R. Schlösser; Universitätsklinikum Hamburg-Eppendorf: D. Singer; Medizinische Hochschule Hannover: B. Bohnhorst; Universitätsmedizin Mainz: A. Kidszun; Klinikum Saarbrücken: J. Möller; Universitätsklinikum Greifswald: M. Heckmann; Klinik für Kinder- und Jugendmedizin Speyer: A. Bosk; Kinderklinik Uniklinik Köln: A. Kribs; Universitätsklinikum Freiburg: R. Hentschel. Greece: Aristotle University - General Hospital Papageorgiou, Thessaloniki: P. Karagianni/K. Serafidis; “Mitera” General Maternity and Pediatric Clinic, Athens: M. Saklamaki-kontou; “Lito” Maternity Hospital, Athens: V. Delivoria; Helena Venizelou Perinatal Center, Athens: H. Salvanos; Alexandras Hospital General, Athens: G. Baroutis. Hungary: Semmelweis University, Budapest: M. Szabo; Medical University of Pécs: T. Erlt; Dél-pesti Kórház és Rendelőintézet, Budapest: M. C. László. Ireland: National University of Ireland, Galway: D. O′ Donovan; University College Cork: E. Dempsey; Coombe Women & Infants University Hospital, Dublin: J. Miletin; Rotunda Hospital, Dublin: N. McCallion. Italy: Azienda Ospedaliera di Padova: D. Trevisanuto; Azienda Ospedaliera Universitaria Pisana: A. Boldrini; Ospedale Maggiore Policlinico-Università degli Studi di Milano: F. Mosca; Ospedale dei Bambini ´V. Buzzi’, Milan: G. Lista; Ospedale Evangelico Villa Betania, Naples: F. Messina; Azienda Ospedaliera Universitaria Federico II, Naples: F. Raimondi; Careggi University Hospital of Florence: C. Dani; University La Sapienza, Rome: M. De Curtis; Ospedale San Giovanni Calibita FBF Isola Tiberina, Rome: L. Orfeo; Ospedale San Gerardo, Monza: P. Tagliabue; P.O. SS Annunziata, Naples: T. Vacchiano; Azienda Ospedaliera di Venere e Giovanni XXIII, Bari: A. del Vecchio. Latvia: Pauls Stradins Clinical University Hospital, Riga: V. Urtans. Lithuania: Hospital of Kaunas University of Medicine: R. Tamelienė; Vilnius University Childrens’ Hospital: A. Liubšys. Malta: Mater Dei Hospital: P. Soler. Moldavia: Institute for Mother and Child Health Care, Chisinau: V. Iesanu. Netherlands: Emma Children’s Hospital, Amsterdam: A. von Kaam; University Hospital Utrecht - Wilhelmina Children’s Hospital: F. Van Bel; Leiden University Medical Centre: A. te Pas; Beatrix Children’s Hospital, University of Groningen: A. Bos; Radboud University Medical Center, Nijmegen: W. de Boode. Norway: Oslo Rikshospitalet University Hospital: O. Saugstad; Barneklinikken Haukeland Sykehus, Bergen: H. Reigstad; University of Tromsø-Arctic, Tromsø: C. Klingenberg; Ålesund Hospital: W. Sivertsen; Vestre Viken Hospital, Drammen: K. Hochnowski; Sykehuset Østfold Hospital, Fredrikstad: S.H. Anderssen; Akershus University Hospital, Lørenskog: B. Nakstad. Poland: Olsztyn Hospital: J. Meller. Portugal: Centro Hospitalar de São João, Porto: H. Guimaraes; Maternidade Bissaya Barreto, Coimbra: C. Resende; Maternidade Daniel Matos, Coimbra: M. Afonso. Romania: “Polizu” Clinical Hospital, Bucharest: S. Stoicescu; Panait Sarbu Hospital, Bucharest: A. Toma; “Cuza Voda” Clinical Hospital of Obstetrics and Gynecology, Lasi: M. Stamatin. Slovakia: Jessenius Medical Faculty Martin/Comenius University Bratislava: M. Zibolen, K. Matasova; Klinika Neonatológie, Kosice: V. Chovanova. Slovenia: University Medical Centre Ljubljana: D. Paro-Panjan; University Medical Center Maribor: M. Treiber. Spain: Hospital General Universitario de Alicante: C. Tapia Collados; Hospital Universitario Príncipe de Asturias: M. Jesus Ripalda; Hospital Clínic de Barcelona: F. Botet; Hospital de Navarra: A. Ocon; Complejo Hospitalario de Pontevedra: P. Suarez; Hospital Universitario de Salamanca: L. San Feliciano; Hospital Universitario Severo Ochoa, Madrid: E. Carrasco; Complejo Hospitalario Universitario de Vigo: A. Guisan; Hospital Virgen de la Salud, Toledo: M. Redondo; University & Polytechnic Hospital La Fe, Valencia: M. Vento; Gregorio Marañon University Hospital, Madrid: M. Sanchez-Luna; Hospital Infantil La Paz, Madrid: F. Omeñaca; Complexo Hospitalario Universitario A Coruña: A. Avila-Alvarez; Hospital Sant Joan de Déu , Barcelona: M. Iriondo-Sanz; Hospital Clinico San Carlos, Madrid: G. Carillo. Sweden: University Hospital, Umeå: S. Håkansson; Uppsala University: L. Hellström-Westas; Karolinska Institutet and University Hospital, Stockholm: K. Bohlin. Switzerland: University of Basel Children’s Hospital: S. Schulzke; Bern University Hospital: M. Nelle; Children’s Hospital Lucerne: M. Stocker; Geneva University Hospital: P. Rimensberger; University Hospital Zurich: C. Hagmann; Ostschweizer Kinderspital, St Gallen: D. Bailey. Turkey: Zekai Tahir Burak Maternity Teaching Hospital, Ankara: G. Kanmaz; Gazi University Hospital, Ankara: E. Ergenekon; Marmara University School of Medicine, Istanbul: E. Ozek; Firat University School of Medicine, Elazig: M. Aydin; Ataturk University Medical Faculty, Erzurum: K. Tekgündüz. Ukraine: Lviv National Medical University: K. Stepanovich. United Kingdom: Royal Maternity Hospital, Queen’s University, Belfast: D. Sweet; Imperial College London: P. Montaldo; Brighton and Sussex Medical School: H. Rabe; University Hospital of North Tees and University of Durham, Stockton-on-Tees: S. Gupta; Norfolk and Norwich University Hospitals: P. Clarke; Northampton General Hospital: F. Thompson; Warrington Hospital: D. Webb; Blackpool Teaching Hospital: C. Rawlingson; Evelina London Children’s Hospital St Thomas: T. Watts; Great Ormond Street Hospital, London: Q. Mok; University Hospital of North Staffordshire, Stoke-on-Trent: K. Palmer; Oxford University Hospitals: C. Roehr.
Authors’ Contributions
Daniel Klotz conceived the study, designed the survey, contributed to data collection, analyzed data, and wrote the first draft of the manuscript. Ugo Porcaro designed the survey, collected data, analyzed data, and edited the manuscript. Thilo Fleck analyzed data, helped with translation, and edited the manuscript. Hans Fuchs contributed in study design, edited the survey, contributed in data collection and analysis, and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No external funding was received for this study.
Ethical approval
The study was approved by the Ethics committee of the University of Freiburg.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Patrick Van Reempts
Rights and permissions
About this article
Cite this article
Klotz, D., Porcaro, U., Fleck, T. et al. European perspective on less invasive surfactant administration—a survey. Eur J Pediatr 176, 147–154 (2017). https://doi.org/10.1007/s00431-016-2812-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00431-016-2812-9