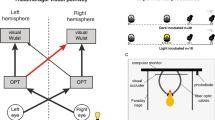
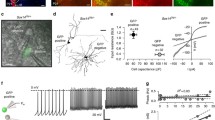
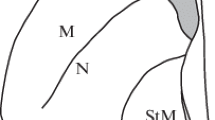
Abstract
The lateralized visual systems of pigeons and chickens are excellent models to study neural asymmetries at the functional and anatomical level. The aim of the current study was to reveal why these two species closely resemble each other with respect to left–right differences in behavior but not with respect to the pathways involved: While pigeons show an asymmetrically organized tectofugal system, only transient lateralizations of the thalamofugal system have been observed in chickens. Four possible explanations are conceivable. (1) Adult pigeons might also show a hitherto undiscovered thalamofugal asymmetry like chickens. (2) The thalamofugal asymmetry might be transient in both species. (3) Prehatch light stimulation could differentially affect the two visual pathways of chickens and pigeons that mature with different speeds. (4) Tecto- and thalamofugal asymmetries represent species differences, independent of developmental factors. To test these explanations, we injected retrograde tracers into the Wulst of adult pigeons, of hatchlings, and of dark reared pigeons which were monocularly deprived on their left or right eye for one week after hatch. Subsequently we counted labeled cells within the ipsi- and contralateral n. geniculatus lateralis pars dorsalis in search for possible lateralizations of ascending pathways. None of the experimental groups displayed significant differences in the thalamofugal projection pattern. This indicates that visual lateralization in pigeons and chickens depends on tectofugal and thalamofugal asymmetries, respectively. Thus, in different species a highly similar pattern of behavioral asymmetries can be subserved by diverse neural systems.









Similar content being viewed by others
References
Andrew RJ, Johnston ANB, Robins A, Rogers LJ (2004) Light experience and the development of behavioral lateralization in chicks. II. Choice of familiar versus unfamiliar model social partner. Behav Brain Res 155:67–76
Bagnoli P, Burkhalter A (1983) Organization of the afferent projections to the Wulst in the pigeon. J Comp Neurol 214:103–113
Buchner A, Erdfelder E, Faul F (1997) How to Use G*Power Heinrich-Heine-Universität Düsseldorf, Institut für Experimentelle Psychologie. http://www.psycho.uni-duesseldorf.de/aap/projects/gpower/how_to_use_gpower.html. Accessed 27 Juli 2012
Chiandetti C, Regolin L, Rogers LJ, Vallortigara G (2005) Effects of light stimulation of embryos on the use of position-specific and object-specific cues in binocular and monocular domestic chicks (Gallus gallus). Behav Brain Res 163:10–17
Chiesa AD, Speranza M, Tommasi L, Vallortigara G (2006) Spatial cognition based on geometry and landmarks in the domestic chick (Gallus gallus). Behav Brain Res 175:119–127
Deng C, Rogers LJ (2002) Factors affecting the development of lateralization in chicks. In: Rogers LJ, Andrew R (eds) Comparative vertebrate lateralization. Cambridge University Press, Cambridge, pp 206–246
Diekamp B, Regolin L, Güntürkün O, Vallortigara G (2005) A left-sided visuospatial bias in birds. Curr Biol 15:372–373
Folta K, Diekamp B, Güntürkün O (2004) Asymmetrical modes of visual bottom-up and top-down integration in the thalamic nucleus rotundus of pigeons. J Neurosci 24:9475–9485
Freund N, Güntürkün O, Manns M (2008) A morphological study of the nucleus subpretectalis of the pigeon. Brain Res Bull 75:491–493
Gagliardo A, Vallortigara G, Nardi D, Bingman VP (2005) A lateralized avian hippocampus: preferential role of the left hippocampal formation in homing pigeon sun compass-based spatial learning. Eur J Neurosci 22:2549–2559
Gaston KE, Gaston MG (1984) Unilateral memory after binocular discrimination training: left hemisphere dominance in the chick? Brain Res 303:190–193
Güntürkün O (1985) Lateralization of visually controlled behavior in pigeons. Physiol Behav 34:575–577
Güntürkün O (1997) Avian visual lateralization: a review. Neuroreport 8:iii-xi
Güntürkün O, Karten HJ (1991) An immunocytochemical analysis of the lateral geniculate complex in the pigeon (Columba livia). J Comp Neurol 314:721–749
Güntürkün O, Kesch S (1987) Visual lateralization during feeding in pigeons. Behav Neurosci 101:433–435
Güntürkün O, Melsbach G, Hörster W, Daniel S (1993) Different sets of afferents are demonstrated by the fluorescent tracers fast blue and rhodamine. J Neurosci Methods 49:103–111
Güntürkün O, Hellmann B, Melsbach G, Prior H (1998) Asymmetries of representation in the visual system of pigeons. Neuroreport 9:4127–4130
Güntürkün O, Verhoye M, De Groof G, Van der Linden A (2012) A 3-dimensional digital atlas of the ascending sensory and the descending motor systems in the pigeon brain. Brain Struct Funct. 2012 [Epub ahead of print]
Hackett SJ, Kimball RT, Reddy S, Bowie RC, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-LH, Harshman J, Huddleston CJ, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T (2008) A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768
Halpern ME, Güntürkün O, Hopkins WD, Rogers LJ (2005) Lateralization of the vertebrate brain: taking the side of model systems. J Neurosci 25:10351–10357
Karten HJ, Hodos W (1967) A stereotaxic atlas of the brain of the pigeon (Columba livia). John Hopkins Press, Baltimore
Köbbert C, Apps R, Bechmann I, Lanciego JL, Mey J, Thanos S (2000) Current concepts in neuroanatomical tracing. Prog Neurobiol 62:327–351
Koshiba M, Nakamura S, Deng C, Rogers LJ (2003) Light-dependent development of asymmetry in the ipsilateral and contralateral thalamofugal visual projections of the chick. Neurosci Lett 336:81–84
Manns M, Güntürkün O (1997) Development of the retinotectal system in the pigeon: a cytoarchitectonic and tracing study with cholera toxin. Anat Embryol 195:539–555
Manns M, Güntürkün O (1999a) Monocular deprivation alters the direction of functional and morphological asymmetries in the pigeon’s (Columba livia) visual system. Behav Neurosci 113:1257–1266
Manns M, Güntürkün O (1999b) ‘Natural’ and artificial monocular deprivation effects on thalamic soma sizes in pigeons. Neuroreport 10:3223–3228
Manns M, Güntürkün O (2003) Light experience induces differential asymmetry pattern of GABA- and parvalbumin-positive cells in the pigeon’s visual midbrain. J Chem Neuroanat 25:249–259
Manns M, Güntürkün O (2009) Dual coding of visual asymmetries in the pigeon brain: the interaction of bottom-up and top-down systems. Exp Brain Res 199:323–332
Manns M, Freund N, Güntürkün O (2008) Development of the diencephalic relay structures of the visual thalamofugal system in pigeons. Brain Res Bull 75:424–427
Mench JA, Andrew RJ (1986) Lateralization of a food search task in the domestic chick. Behav Neural Biol 46:107–114
Miceli D, Peyrichoux J, Repérant J (1975) The retino-thalamo-hyperstriatal pathway in the pigeon (Columba livia). Brain Res 100:125–131
Miceli D, Marchand L, Repérant J, Rio JP (1990) Projections of the dorsolateral anterior complex and adjacent thalamic nuclei upon the visual Wulst in the pigeon. Brain Res 518:317–323
Nagy M, Akosm Z, Biro D, Vicsek T (2010) Hierarchical group dynamics in pigeon flocks. Nature 464:890–893
Nardi D, Bingman VP (2007) Asymmetrical participation of the left and right hippocampus for representing environmental geometry in homing pigeons. Behav Brain Res 178:160–171
Prior H, Güntürkün O (2001) Parallel working memory for spatial location and food-related object cues in foraging pigeons: binocular and lateralized monocular performance. Learn Mem 8:44–51
Prior H, Lingenauber F, Nitschke J, Güntürkün O (2002) Orientation and lateralized cue use in pigeons navigating a large indoor environment. J Exp Biol 205:1795–1805
Prior H, Wiltschko R, Stapput K, Güntürkün O, Wiltschko W (2004) Visual lateralization and homing in pigeons. Behav Brain Res 154:301–310
Rogers LJ (1982) Light experience and asymmetry of brain function in chickens. Nature 297:223–225
Rogers LJ (1990) Light input and the reversal of functional lateralization in the chicken brain. Behav Brain Res 38:211–221
Rogers LJ (2002) Advantages and disadvantages of lateralization. In: Rogers LJ, Andrew R (eds) Comparative vertebrate lateralization. Cambridge University Press, Cambridge, pp 126–154
Rogers LJ (2008) Development and function of lateralization in the avian brain. Brain Res Bull 76:235–244
Rogers LJ, Bell GA (1989) Different rates of functional development in the two visual systems of the chicken revealed by [14C]2-deoxyglucose. Brain Res Dev Brain Res 49:161–172
Rogers LJ, Bolden SW (1991) Light-dependent development and asymmetry of visual projections. Neurosci Lett 121:63–67
Rogers LJ, Deng C (1999) Light experience and lateralization of the two visual pathways in the chick. Behav Brain Res 98:277–287
Rogers LJ, Rajendra S (1993) Modulation of the development of light-initiated asymmetry in chick thalamofugal visual projections by oestradiol. Exp Brain Res 93:89–94
Rogers LJ, Sink HS (1988) Transient asymmetry in the projections of the rostral thalamus to the visual hyperstriatum of the chicken, and reversal of its direction by light exposure. Exp Brain Res 70:378–384
Rosa Salva O, Regolin L, Vallortigara G (2007) Chicks discriminate human gaze with their right hemisphere. Behav Brain Res 177:15–21
Siegel JJ, Nitz D, Bingman VP (2006) Lateralized functional components of spatial cognition in the avian hippocampal formation: evidence from single-unit recordings in freely moving homing pigeons. Hippocampus 16:125–140
Skiba M, Diekamp B, Güntürkün O (2002) Embryonic light stimulation induces different asymmetries in visuoperceptual and visuomotor pathways of pigeons. Behav Brain Res 134:149–156
Tommasi L, Vallortigara G (2001) Encoding of geometric and landmark information in the left and right hemispheres of the Avian Brain. Behav Neurosci 115:602–613
Tommasi L, Vallortigara G (2004) Hemispheric processing of landmark and geometric information in male and female domestic chicks (Gallus gallus). Behav Brain Res 155:85–96
Vallortigara G, Rogers LJ (2005) Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28:575–589
Vallortigara G, Regolin L, Bortolomiol G, Tommasi L (1996) Lateral asymmetries due to preferences in eye use during visual discrimination learning in chicks. Behav Brain Res 74:135–143
Vallortigara G, Cozzutti C, Tommasi L, Rogers LJ (2001) How birds use their eyes: opposite left-right specialization for the lateral and frontal visual hemifield in the domestic chick. Curr Biol 11:29–33
Vallortigara G, Pagni P, Sovrano VA (2004) Separate geometric and non-geometric modules for spatial reorientation: evidence from a lopsided animal brain. J Cogn Neurosci 16:390–400
Verhaal J, Kirsch J, Vlachos I, Manns M, Güntürkün O (2012) Lateralized reward-related visual discrimination in the avian entopallium. Eur J Neurosci 35:1337–1343
von Fersen L, Güntürkün O (1990) Visual memory lateralization in pigeons. Neuropsychologia 28:1–7
Wilzeck C, Prior H, Kelly DM (2009) Geometry and landmark representation by pigeons: evidence for species-differences in the hemispheric organization of spatial information processing? Eur J Neurosci 29:813–822
Yamazaki Y, Aust U, Huber L, Hausmann M, Güntürkün O (2007) Lateralized cognition: asymmetrical and complementary strategies of pigeons during discrimination of the “human concept”. Cognition 104:315–344
Acknowledgments
We thank Arianne Schwarz for her help with immunohistochemical staining and Josine Verhaal as well as Sabine Kesch for their help with animal breeding. This research was supported by a DFG grant from the German Science Foundation DFG to M.M. and to O.G. (SFB 874).
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Ströckens and N. Freund contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ströckens, F., Freund, N., Manns, M. et al. Visual asymmetries and the ascending thalamofugal pathway in pigeons. Brain Struct Funct 218, 1197–1209 (2013). https://doi.org/10.1007/s00429-012-0454-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00429-012-0454-x