Abstract
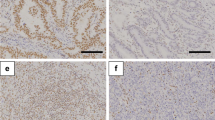
This study was designed to unravel the pathobiological role of impaired ARID1A expression in gastric carcinogenesis. We examined ARID1A expression immunohistochemically in 98 gastric cancer tissue specimens with regard to the clinicopathological features. Based on the proportion and intensity of ARID1A immunoreactivity at the cancer invasion front, we subdivided the specimens into low- and high-expression ARID1A groups. Notably, low ARID1A expression was significantly correlated with overall survival of the patients. Subsequently, we determined the molecular signature that distinguished ARID1A low/poor prognosis from ARID1A high/good prognosis gastric cancers. A comprehensive gene profiling analysis followed by immunoblotting revealed that a mitochondrial apoptosis mediator, Harakiri, was less expressed in ARID1A low/poor prognosis than ARID1A high/good prognosis gastric cancers. siRNA-mediated ARID1A downregulation significantly reduced expression of the Harakiri molecule in cultured gastric cancer cells. Interestingly, downregulation of ARID1A conferred resistance to apoptosis induced by the mitochondrial metabolism inhibitor, devimistat. In contrast, enforced Harakiri expression restored sensitivity to devimistat-induced apoptosis in ARID1A downregulated gastric cancer cells. The present findings indicate that impaired ARID1A expression might lead to gastric carcinogenesis, putatively through gaining resistance to the Harakiri-mediated apoptosis pathway.



Similar content being viewed by others
Data availability
The microarray data have been deposited to the GEO database with accession number GSE110078.
References
de la Serna IL, Ohkawa Y, Imbalzano AN (2006) Chromatin remodelling in mammalian differentiation: lessons from ATP-dependent remodellers. Nat Rev Genet 7:461–473. https://doi.org/10.1038/nrg1882
Luger K, Dechassa ML, Tremethick DJ (2012) New insights into nucleosome and chromatin structure: an ordered state or a disordered affair? Nat Rev Mol Cell Biol 13:436–447. https://doi.org/10.1038/nrm3382
Takeuchi T, Chen BK, Qiu Y, Sonobe H, Ohtsuki Y (1997) Molecular cloning and expression of a novel human cDNA containing CAG repeats. Gene 204:71–77. https://doi.org/10.1016/s0378-1119(97)00525-8
Nie Z, Xue Y, Yang D, Zhou S, Deroo BJ, Archer TK, Wang W (2000) A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol Cell Biol 20:8879–8888. https://doi.org/10.1128/mcb.20.23.8879-8888.2000
Dallas PB, Pacchione S, Wilsker D, Bowrin V, Kobayashi R, Moran E (2000) The human SWI-SNF complex protein p270 is an ARID family member with non-sequence-specific DNA binding activity. Mol Cell Biol 20:3137–3146. https://doi.org/10.1128/mcb.20.9.3137-3146.2000
Kato H, Tjernberg A, Zhang W, Krutchinsky AN, An W, Takeuchi T, Ohtsuki Y, Sugano S, de Bruijn DR, Chait BT, Roeder RG (2002) SYT associates with human SNF/SWI complexes and the C-terminal region of its fusion partner SSX1 targets histones. J Biol Chem 277:5498–5505. https://doi.org/10.1074/jbc.M108702200
Wilsker D, Patsialou A, Dallas PB, Moran E (2002) ARID proteins: a new family of DNA binding proteins implicated in the control of cell growth, differentiation, and development. Cell Growth Differ 13:95–106
Gao X, Tate P, Hu P, Tjian R, Skarnes WC, Wang Z (2008) ES cell pluripotency and germ-layer formation require the SWI/SNF chromatin remodeling component BAF250a. Proc Natl Acad Sci U S A 105:6656–6661. https://doi.org/10.1073/pnas.0801802105
Takeuchi T, Nicole S, Misaki A, Furihata M, Iwata J, Sonobe H, Ohtsuki Y (2001) Expression of SMARCF1, a truncated form of SWI1, in neuroblastoma. Am J Pathol 158:663–672. https://doi.org/10.1016/S0002-9440(10)64008-4
Jones S, Wang TL, Shih IM, Mao TL, Nakayama K, Roden R, Glas R, Slamon D, Diaz LA Jr, Vogelstein B, Kinzler KW, Velculescu VE, Papadopoulos N (2010) Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330:228–231. https://doi.org/10.1126/science.1196333
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, Yang W, Heravi-Moussavi A, Giuliany R, Chow C, Fee J, Zayed A, Prentice L, Melnyk N, Turashvili G, Delaney AD, Madore J, Yip S, McPherson AW, Ha G, Bell L, Fereday S, Tam A, Galletta L, Tonin PN, Provencher D, Miller D, Jones SJ, Moore RA, Morin GB, Oloumi A, Boyd N, Aparicio SA, Shih IM, Mes-Masson AM, Bowtell DD, Hirst M, Gilks B, Marra MA, Huntsman DG, Wiegand KC (2010) ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med 363:1532–1543. https://doi.org/10.1056/NEJMoa1008433
Wiegand KC, Lee AF, Al-Agha OM, Chow C, Kalloger SE, Scott DW, Steidl C, Wiseman SM, Gascoyne RD, Gilks B, Huntsman DG (2011) Loss of BAF250a (ARID1A) is frequent in high-grade endometrial carcinomas. J Pathol 224:328–333. https://doi.org/10.1002/path.2911
Guan B, Wang TL, Shih IM (2011) ARID1A, a factor that promotes gynecologic cancers. Cancer Res 71:6718–6727. https://doi.org/10.1158/0008-5472.CAN-11-1562
Kandoth C, McLellan MD, Vandin F, Ye K, Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, Leiserson MDM, Miller CA, Welch JS, Walter MJ, Wendl MC, Ley TJ, Wilson RK, Raphael BJ, Ding L (2013) Mutational landscape and significance across 12 major cancer types. Nature 502:333–339. https://doi.org/10.1038/nature12634
Takeuchi T, Misaki A, Liang SB, Tachibana A, Hayashi N, Sonobe H, Ohtsuki Y (2000) Expression of T-cadherin (CDH13, H-cadherin) in human brain and its characteristics as a negative growth regulator of epidermal growth factor in neuroblastoma cells. J Neurochem 74:1489–1497. https://doi.org/10.1046/j.1471-4159.2000.0741489.x
Takao C, Morikawa A, Ohkubo H, Kito Y, Saigo C, Sakuratani T, Futamura M, Takeuchi T, Yoshida K (2017) Downregulation of ARID1A, a component of the SWI/SNF chromatin remodeling complex, in breast cancer. J Cancer 8:1–8. https://doi.org/10.7150/jca.16602
Akiyama S, Amo H, Watanabe T, Matsuyama M, Sakamoto J, Imaizumi M, Ichihashi H, Kondo T, Takagi H (1988) Characteristics of three human gastric cancer cell lines, NU-GC-2, NU-GC-3 and NU-GC-4. Jpn J Surg 18:438–446. https://doi.org/10.1007/bf02471470
Sakakibara K, Suzuki T, Motoyama T, Watanabe H, Nagai Y (1982) Biosynthesis of an interstitial type of collagen by cloned human gastric carcinoma cells. Cancer Res 42:2019–2027
Murakami H, Nakanishi H, Tanaka H, Ito S, Misawa K, Ito Y, Ikehara Y, Kondo E, Kodera Y (2013) Establishment and characterization of novel gastric signet-ring cell and non signet-ring cell poorly differentiated adenocarcinoma cell lines with low and high malignant potential. Gastric Cancer 16:74–83. https://doi.org/10.1007/s10120-012-0149-2
Takeuchi T, Adachi Y, Nagayama T (2012) WWOX-binding molecule, transmembrane protein 207, is related to the invasiveness of gastric signet-ring cell carcinoma. Carcinogenesis. 33:548–554. https://doi.org/10.1093/carcin/bgs001
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76:4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Miyata K, Yasukawa T, Fukuda M, Takeuchi T, Yamazaki K, Sakumi K, Tamamori-Adachi M, Ohnishi Y, Ohtsuki Y, Nakabeppu Y, Kitajima S, Onishi S, Aso T (2007) Induction of apoptosis and cellular senescence in mice lacking transcription elongation factor, Elongin A. Cell Death Differ 14:716–726. https://doi.org/10.1038/sj.cdd.4402067
Inohara N, Ding L, Chen S, Núñez G (1997) harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L). EMBO J 16:1686–1694. https://doi.org/10.1093/emboj/16.7.1686
Xu M, Chen X, Chen N, Nie L, Li X, Li Q, Zeng H, Zhou Q (2013) Synergistic silencing by promoter methylation and reduced AP-2α transactivation of the proapoptotic HRK gene confers apoptosis resistance and enhanced tumor growth. Am J Pathol 182:84–95. https://doi.org/10.1016/j.ajpath.2012.09.018
Kaya-Aksoy E, Cingoz A, Senbabaoglu F, Seker F, Sur-Erdem I, Kayabolen A, Lokumcu T, Sahin GN, Karahuseyinoglu S, Bagci-Onder T (2019) The pro-apoptotic Bcl-2 family member Harakiri (HRK) induces cell death in glioblastoma multiforme. Cell Death Discov 5:64. https://doi.org/10.1038/s41420-019-0144-z
Lee KC, Shorr R, Rodriguez R, Maturo C, Boteju LW, Sheldon A (2011) Formation and anti-tumor activity of uncommon in vitro and in vivo metabolites of CPI-613, a novel anti-tumor compound that selectively alters tumor energy metabolism. Drug Metab Lett 5:163–182. https://doi.org/10.2174/187231211796904991
Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, Schauble A, Lem J, Piramzadian A, Karnik S, Lee K, Rodriguez R, Shorr R, Bingham PM (2011) Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med (Berl) 89:1137–1148. https://doi.org/10.1007/s00109-011-0785-8
Wiegand KC, Sy K, Kalloger SE, Li-Chang H, Woods R, Kumar A, Streutker CJ, Hafezi-Bakhtiari S, Zhou C, Lim HJ, Huntsman DG, Clarke B, Schaeffer DF (2014) ARID1A/BAF250a as a prognostic marker for gastric carcinoma: a study of 2 cohorts. Hum Pathol 45:1258–1268. https://doi.org/10.1016/j.humpath.2014.02.006
Lee SY, Kim DW, Lee HS, Ihn MH, Oh HK, Park DJ, Kim HH, Kang SB (2015) Loss of AT-rich interactive domain 1A expression in gastrointestinal malignancies. Oncology. 88:234–240. https://doi.org/10.1159/000369140
Kim YB, Ham IH, Hur H, Lee D (2016) Various ARID1A expression patterns and their clinical significance in gastric cancers. Hum Pathol 49:61–70. https://doi.org/10.1016/j.humpath.2015.10.008
Abe H, Maeda D, Hino R, Otake Y, Isogai M, Ushiku AS, Matsusaka K, Kunita A, Ushiku T, Uozaki H, Tateishi Y, Hishima T, Iwasaki Y, Ishikawa S, Fukayama M (2012) ARID1A expression loss in gastric cancer: pathway-dependent roles with and without Epstein-Barr virus infection and microsatellite instability. Virchows Arch 461:367–377. https://doi.org/10.1007/s00428-012-1303-2
Wang K, Kan J, Yuen ST, Shi ST, Chu KM, Law S, Chan TL, Kan Z, Chan AS, Tsui WY, Lee SP, Ho SL, Chan AK, Cheng GH, Roberts PC, Rejto PA, Gibson NW, Pocalyko DJ, Mao M, Xu J, Leung SY (2011) Exome sequencing identifies frequent mutation of ARID1A in molecular subtypes of gastric cancer. Nat Genet 43:1219–1223. https://doi.org/10.1038/ng.982
Luo Q, Wu X, Chang W, Zhao P, Zhu X, Chen H, Nan Y, Luo A, Zhou X, Su D, Jiao W, Liu Z (2020) ARID1A Hypermethylation disrupts transcriptional homeostasis to promote squamous cell carcinoma progression. Cancer Res 80:406–417. https://doi.org/10.1158/0008-5472.CAN-18-2446
Jiang ZH, Peng T, Qian HL, Lu CD, Qiu F, Zhang SZ (2019) DNA damage-induced activation of ATM promotes β-TRCP-mediated ARID1A ubiquitination and destruction in gastric cancer cells. Cancer Cell Int 19:162. https://doi.org/10.1186/s12935-019-0878-y
Obata T, Toyota M, Satoh A, Sasaki Y, Ogi K, Akino K, Suzuki H, Murai M, Kikuchi T, Mita H, Itoh F, Issa JP, Tokino T, Imai K (2003) Identification of HRK as a target of epigenetic inactivation in colorectal and gastric cancer. Clin Cancer Res 9:6410–6418
Kim TH, Yoo JY, Wang Z, Lydon JP, Khatri S, Hawkins SM, Leach RE, Fazleabas AT, Young SL, Lessey BA, Ku BJ, Jeong JW (2015) ARID1A is essential for endometrial function during early pregnancy. PLoS Genet 11(9):e1005537. https://doi.org/10.1371/journal.pgen.1005537
Acknowledgments
We thank Dr. Hayao Nakanishi at the Division of Oncological Pathology, Aichi Cancer Center Research Institute, for the kind gift of GPM-2 gastric cancer cells.
Contributions
TS: analyzing data, collecting data, preparing manuscript
YI, IY: analyzing data, technical support, preparing manuscript
CS, YK: technical support
TT and KY: study design, collecting data, analyzing data, preparing manuscript
Funding
This study was supported by grants from the Ministry of Education of Japan (grant nos. 20K07406, KAKEN 15K08361, 15K19051).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
This study was approval of the Institutional Review Board of the Gifu University Graduate School of Medicine (specific approval numbers: 28-482 and 28-523). Informed consent was obtained from all participants or authorized representatives. The present study was conducted in accordance with the ethical standards of the 1975 Helsinki Declaration.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sakuratani, T., Takeuchi, T., Yasufuku, I. et al. Downregulation of ARID1A in gastric cancer cells: a putative protective molecular mechanism against the Harakiri-mediated apoptosis pathway. Virchows Arch 478, 401–411 (2021). https://doi.org/10.1007/s00428-020-02899-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-020-02899-1