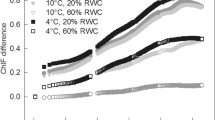
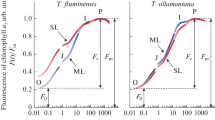
Abstract
In order to survive sunlight in the absence of water, desiccation-tolerant green plants need to be protected against photooxidation. During drying of the chlorolichen Cladonia rangiformis and the cyanolichen Peltigera neckeri, chlorophyll fluorescence decreased and stable light-dependent charge separation in reaction centers of the photosynthetic apparatus was lost. The presence of light during desiccation increased loss of fluorescence in the chlorolichen more than that in the cyanolichen. Heating of desiccated Cladonia thalli, but not of Peltigera thalli, increased fluorescence emission more after the lichen had been dried in the light than after drying in darkness. Activation of zeaxanthin-dependent energy dissipation by protonation of the PsbS protein of thylakoid membranes was not responsible for the increased loss of chlorophyll fluorescence by the chlorolichen during drying in the light. Glutaraldehyde inhibited loss of chlorophyll fluorescence during drying. Desiccation-induced loss of chlorophyll fluorescence and of light-dependent charge separation are interpreted to indicate activation of a highly effective mechanism of photoprotection in the lichens. Activation is based on desiccation-induced conformational changes of a pigment–protein complex. Absorbed light energy is converted into heat within a picosecond or femtosecond time domain. When present during desiccation, light interacts with the structural changes of the protein providing increased photoprotection. Energy dissipation is inactivated and structural changes are reversed when water becomes available again. Reversibility of ultra-fast thermal dissipation of light energy avoids photo-damage in the absence of water and facilitates the use of light for photosynthesis almost as soon as water becomes available.





Similar content being viewed by others
Abbreviations
- F o :
-
Basal modulated chlorophyll fluorescence of hydrated photoautotrophs indcating oxidation of the primary quinone acceptor QA of PSII
- F m :
-
Maximum modulated chlorophyll fluorescence elicited by saturating light pulses indicating reduction of the primary quinone acceptor QA of PSII
- ΔF/F m :
-
(F m − F o)/F m: Quantum efficiency of stable charge separation in PSII
- PPFD:
-
Photosynthetically active photon flux density
- PSII, PSI:
-
Photosystems II or I
References
Alpert P (2000) The discovery, scope and puzzle of desiccation tolerance in plants. Plant Ecol 151:5–17
Aubert S, Juge C, Boisson A-M, Gout E, Bligny R (2007) Metabolic processes sustaining the reviviscence of lichen Xanthoria elegans (Link) in high mountain environments. Planta 226:1287–1297
Bilger W, Rimke S, Schreiber U, Lange OL (1989) Inhibition of energy transfer to photosystem II in lichens by dehydration: different properties of reversibility with green and blue–green photobionts. J Plant Physiol 134:261–268
Coughlan SJ, Schreiber U (1984) The differential effects of short-time glutaraldehyde treatments on light-induced thylakoid membrane conformational changes, proton pumping and electron transport properties. Biochim Biophys Acta 767:606–617
Demmig-Adams B (1990) Carotenoids and photoprotection of plants: a role for the xanthophyll zeaxanthin. Biochim Biophys Acta 1020:1–24
Demmig-Adams B, Máguas C, Adams WWIII, Meyer A, Kilian E, Lange OL (1990) Effect of high light on the efficiency of photochemical energy conversion in a variety of lichen species with green and blue–green phycobionts. Planta 180:400–409
Heber U, Shuvalov VA (2005) Photochemical reactions of chlorophyll in dehydrated photosystem II: two chlorophyll forms (680 and 700 nm). Photosynth Res 84:85–91
Heber U, Lange O-L, Shuvalov VA (2006a) Conservation and dissipation of light energy by plants as complementary processes involved in sustaining plant life: homoiohydric and poikilohydric autotrophs. J Exp Bot 57:1211–1223
Heber U, Bilger W, Shuvalov VA (2006b) Thermal energy dissipation in reaction centers of photosystem II protects desiccated poikilohydric mosses against photooxidation. J Exp Bot 57:2993–3006
Heber U, Azarkovich M, Shuvalov (2007) Activation of mechanisms of photoprotection by desiccation and by light: poikilohydric photoautotrophs. J Exp Bot 58: 2745–2759
Holt NE, Tigmantas D, Valkunas L, Li X-P, Niyogi KK, Fleming GR (2005) Carotenoid cation formation and the regulation of photosynthetic light harvesting. Science 307:433–436
Holzwarth AR, Muller MG, Reus M, Nowazyk M, Saner J, Rogner M (2006) Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: pheophytin is the primary electron acceptor. Proc Natl Acad Sci USA 103:6895–6900
Katona E, Neimanis S, Schönknecht G, Heber U (1992) Photosystem I-dependent cyclic electron transport is important in controlling photosystem II activity in leaves under conditions of water stress. Photosynth Res 34:449–464
Krieger-Liszkay A (2005) Singlet oxygen production in photosynthesis. J Exp Bot 56:337–346
Li X-P, Gilmore AM, Caffari S, Bassi R, Golan T, Kramer D, Niyogi KK (2004) Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J Biol Chem 279:22866–22874
Pascal AA, Liu Z, Broess K, van Oort B, van Amerongen H, Wang C, Horton P, Robert B, Chang W, Ruban A (2005) Molecular basis of photoprotection and control of photosynthetic light-harvesting. Nature 436:134–137
Proctor MCF, Tuba Z (2002) Poikilohydry and homoihydry: antithesis or spectrum of possibilities. New Phytol 156:327–349
Raven PH, Evert RF, Eichhorn SE (1992) Biology of plants. Worth Publishers, New York
Ruban AV, Berera R, Ilioaia C, van Stokkum IHM, Kennis JTM, Pascal AA, van Amerongen H, Robert B, Horton P, van Grondelle R (2007) Identification of a mechanism of photoprotective energy dissipation in higher plants. Nature 450:575–578
Schreiber U, Schliwa U, Bilger W (1986) Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth Res 10:51–62
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochim Biophys Acta 1767:1233–1244
Veerman J, Vasil’ev S, Paton GD, Ramanauskas J, Bruce D (2007) Photoprotection in the lichen Parmelia sulcata: The origins of desiccation-induced fluorescence quenching. Plant Physiol 145:997–1005
Yamamoto HY, Kamite L (1972) The effects of dithiothreitol on violaxanthin de-epoxidation and absorbance changes in the 500 nm region. Biochim Biophys Acta 267:538–543
Zinth W, Kaiser W (1993) Time-resolved spectroscopy of the primary electron transfer in reaction centers of Rhodopacter sphaeroides and Rhodopseudomonas viridis. In: Deisenhofer J, Norris JR (eds) The photosynthetic reaction center. Academic Press, San Diego, pp 71–88
Acknowledgments
I am grateful to anonymous reviewers who have read and criticized a first version of this contribution. Special thanks are due to reviewer 4. Professor R. Hedrich, head of the chair of Molecular Plant Physiology and Biophysics of the Julius-von-Sachs-Institute of the University of Würzburg, has provided laboratory space and facilities.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Professor Otto Ludwig Lange, Würzburg, on the occasion of his 80th birthday.
Rights and permissions
About this article
Cite this article
Heber, U. Photoprotection of green plants: a mechanism of ultra-fast thermal energy dissipation in desiccated lichens. Planta 228, 641–650 (2008). https://doi.org/10.1007/s00425-008-0766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-008-0766-5