Abstract
An inverse relationship exists between striated muscle fiber size and its oxidative capacity. This relationship implies that muscle fibers, which are triggered to simultaneously increase their mass/strength (hypertrophy) and fatigue resistance (oxidative capacity), increase these properties (strength or fatigue resistance) to a lesser extent compared to fibers increasing either of these alone. Muscle fiber size and oxidative capacity are determined by the balance between myofibrillar protein synthesis, mitochondrial biosynthesis and degradation. New experimental data and an inventory of critical stimuli and state of activation of the signaling pathways involved in regulating contractile and metabolic protein turnover reveal: (1) higher capacity for protein synthesis in high compared to low oxidative fibers; (2) competition between signaling pathways for synthesis of myofibrillar proteins and proteins associated with oxidative metabolism; i.e., increased mitochondrial biogenesis via AMP-activated protein kinase attenuates the rate of protein synthesis; (3) relatively higher expression levels of E3-ligases and proteasome-mediated protein degradation in high oxidative fibers. These observations could explain the fiber type–fiber size paradox that despite the high capacity for protein synthesis in high oxidative fibers, these fibers remain relatively small. However, it remains challenging to understand the mechanisms by which contractile activity, mechanical loading, cellular energy status and cellular oxygen tension affect regulation of fiber size. Therefore, one needs to know the relative contribution of the signaling pathways to protein turnover in high and low oxidative fibers. The outcome and ideas presented are relevant to optimizing treatment and training in the fields of sports, cardiology, oncology, pulmonology and rehabilitation medicine.
Similar content being viewed by others
Introduction
Three decades ago, it was first demonstrated in humans that training at the same time for both strength and endurance results in less adaptation of skeletal muscles compared to training for either one alone, a phenomenon known as the concurrent training effect (Hickson 1980). Generally, resistance exercise leads to an increase in muscle mass (hypertrophy), whereas endurance exercise is associated with increased oxidative metabolism (mitochondrial and capillary density, oxidative enzymes) and no hypertrophy. Why do muscles have limited capacity to increase strength (i.e., fiber size) and endurance capacity (i.e., oxidative metabolism) at the same time? To clarify the relationship between the regulation of fiber size and oxidative metabolism, the present review will focus on the potential cellular and molecular mechanisms underlying the size differences in high and low oxidative muscle fibers. The results and concepts presented in this review are not only relevant to cell and molecular biologists with interest in muscle physiology, but also to the fields of sports and rehabilitation medicine where they are important for understanding physiological limitations in performance (e.g., in patients suffering from chronic diseases) and can be helpful in developing strategies to improve performance.
Virtually, all vertebrate body movements are generated by coordinated activity of skeletal muscles with varying contractile properties, which are governed by the muscle phenotype. Muscle phenotype is the result of interaction between genotype and external influences on muscle fibers and is largely defined by the number of muscle fibers, the fiber cross-sectional area, the number of sarcomeres in series, the muscle fiber type distribution and muscle architecture. Muscle fiber types are generally distinguished according to the predominantly expressed isoform of myosin heavy chain (MyHC), which are referred to as type I, IIA, IIX and IIB. Muscle fibers have the ability to adapt their phenotype and modify MyHC-type, architecture and their size in response to changes in internal or external environment (Pette and Staron 2001).
The maximum rates of oxygen consumption (VO2max) per volume unit and the cross-sectional areas of striated myocytes from different vertebrates vary over a 100-fold range. Muscle fibers with a high oxidative capacity are relatively small compared to fibers with a low oxidative capacity (Van Der Laarse et al. 1998), pointing to an inverse relationship between fiber cross-sectional area and VO2max (Fig. 1). A Hill-type model for oxygen diffusion (Hill 1965) predicts that the maximum value of the product, cross-sectional area × oxygen consumption, is limited by the extracellular oxygen tension as given by:
where VO2max is the maximum rate of oxygen consumption in (nmol mm−3 s−1), CSA is the cross-sectional area of the myocyte (in mm2), α M is the solubility of oxygen in muscle (in mM mmHg−1), DO2 is the diffusion coefficient for oxygen in sarcoplasm (in mm2 s−1), and PO2 (in mmHg) equals the interstitial oxygen tension. If the value of the left-hand term of the equation is larger than that of the right-hand term, an anoxic core in the muscle cell will develop when the cell is maximally activated, causing a reduction in the rate of oxidative ATP production. Thus, any increase in either CSA or VO2max (or both) would require a proportional increase in extracellular PO2 above the value at which the core of the cell becomes anoxic at the maximum rate of oxygen consumption (PO2crit). Experimental determination of α M × DO2 (Krogh’s diffusion coefficient) at VO2max (van der Laarse et al. 2005) validated the Hill model and allowed estimating that striated muscle cells are evolved to function at their maximum rate of oxygen consumption at an extracellular PO2 of about 14 mmHg.
Maximum rate of oxygen consumption (VO2max in nmol mm−3 s−1) of muscle preparations at physiological temperature from various species plotted against the cross-sectional area (in μm2) of the muscle cells in the preparation. A hyperbola was fitted through all data points. The fit hardly deviates from the Hill-type diffusion model shown in the text and is described by the function VO2max = constant CSA−1. The value of the constant is calculated as the mean of the products VO2max and CSA for each muscle fiber type and approximates 0.4 pmol mm−1 s−1. Inset cross sections stained for succinate dehydrogenase activity. From left to right: right ventricular wall of normal rat myocardium, rat extensor digitorum longus muscle, human vastus lateralis muscle, iliofibularis muscle of Xenopus laevis; scale bar 100 μm. ratMCT right ventricular rat cardiomyocytes of a monocrotaline-induced pulmonary hypertensive rat, humanCHF vastus lateralis muscle of human chronic heart failure patients. Figure adapted from (Bekedam et al. 2003; Van Der Laarse et al. 1998)
It is worth noting that, on one hand, VO2max is proportional to succinate dehydrogenase (SDH) activity (Bekedam et al. 2003; Des Tombe et al. 2002; van der Laarse et al. 1989) or oxoglutarate dehydrogenase activity (Blomstrand et al. 1997) and consequently to the number of mitochondria (Hoppeler and Billeter 1991; Reichmann et al. 1991), while several studies consistently showed that muscle fibers with a relatively large cross-sectional area had low SDH activities and vice versa (e.g., Kayar and Banchero 1987; Rivero et al. 1999; Smith and Ovalle 1973). These last results, however, are not normalized for optical density per time unit, fiber volume and physiological temperature.
The relationship between cross-sectional area and mitochondrial density implies that a muscle fiber can hypertrophy (and become stronger) at the expense of its maximum steady state power per fiber volume (i.e., endurance capacity). To prevent a decline in maximum steady state power, oxygen supply to the mitochondria should be improved, for instance by:
-
1.
increasing oxygen transport to the muscle fiber, e.g., by increasing myoglobin, capillary density, hematocrit or a combination of these (Des Tombe et al. 2002; Hickson and Rosenkoetter 1981; van Beek-Harmsen et al. 2004)
-
2.
reducing the diffusion distance for oxygen to the mitochondria by relocating the mitochondria to the sarcolemma (Deveci et al. 2001; Hardy et al. 2009). However, this would imply large radial gradients of (phosphoryl)creatine, Pi and ΔG ATP, which can be expected to have functional consequences.
Although it may be feasible to increase mitochondrial density while fiber size remains similar or even increases, these adaptations are associated with an increase in the number of capillaries per fiber, hence increase in capacity for oxygen supply (Bigard et al. 1991; Desplanches et al. 1996; Deveci et al. 2001; Green et al. 1999). However, to be able to extract oxygen from the blood and to prevent an anoxic core, PO2crit of muscle fibers must be lower than end capillary PO2, which equals 15–20 mmHg in exercising humans (Calbet et al. 2009). This is close to the calculated PO2crit. Increasing PO2crit to a value approaching end capillary PO2 would require an exponential increase in the number of capillaries per muscle fiber to prevent hypoxic cores, which, to the best of our knowledge, has not been reported. Therefore, the smaller size of high oxidative muscle fibers can be considered an evolutionary design constraint.
It should be realized that the classification of types based on MyHC (I, IIA, IIX and IIB) does not necessarily correlate with the oxidative capacity of the muscle fiber. In general, type IIB/IIX fibers have a relatively low oxidative capacity and a large fiber size compared to type I fibers. However, when comparing the oxidative capacity and fiber size between type IIA and type I fibers, literature shows less distinctive results. For example in men, it appears that type I fibers have relatively small CSA compared to type IIA fibers (~5,000 vs. ~7,000 μm2), while their oxidative capacity (as determined by SDH) is significantly higher (Gregory et al. 2001). Also, Bekedam et al. (2003) showed that human type I fibers showed smaller CSA and significantly higher SDH activities compared to type II fibers (all isoforms). However, in women, type I fibers are often larger than type IIA fibers; in rat skeletal muscle, it has been shown that type I fibers show similar or even larger CSA compared to type IIA fibers, while their SDH activities are significantly lower (Nakatani et al. 1999; Simoneau and Bouchard 1989; Wust et al. 2009). Thus, it may well be that type I fibers are larger than type IIA fibers and may be capable of hypertrophy under various conditions and training regimes (e.g., Alway et al. 1988; Andersen et al. 2008; Ianuzzo et al. 1989). However, though the differences in size and oxidative capacity between type I and IIA fibers may be too small to detect experimentally in humans, we hypothesize, based on the data in Fig. 1, that the larger type I fibers would have relatively low oxidative capacities compared to the IIA fibers. Based on the current literature, it is generally safe to conclude that both type I and IIA fibers have a relatively large oxidative capacity and small fiber size compared to type IIB/IIX fibers. In addition, these data also strengthen the observation from Fig. 1 that the fiber size (and not necessarily the fiber type) is related to its oxidative capacity.
The central question addressed in this review is: why do high oxidative muscle fibers remain relatively small compared to low oxidative muscle fibers? To gain insight into the regulation of fiber size and the oxidative metabolism and to reveal the gaps in the current knowledge, we will give a quantitative inventory of fiber type-related differences in the structures and systems involved in synthesis and degradation of muscle protein, as well as an overview of the molecular pathways responsible for hypertrophy and oxidative metabolism and of the way in which way these pathways interact.
Fiber type-related differences in the machinery for protein turnover
In skeletal muscle, there is continuous turnover of proteins, and the balance between protein synthesis and degradation (human cellular protein turnover: ~0.8 g kg body weight−1 day−1 (Paddon-Jones and Rasmussen 2009) determines how the functional parameters change, i.e., whether contractile or mitochondrial protein is gained or lost. Muscle protein synthesis involves both the genetic expression of contractile and mitochondrial proteins themselves and the expression and activation of co-factors controlling expression of these proteins. The expression of contractile and mitochondrial proteins is controlled by both the mitochondrial and the nuclear genome. The synthesis machinery comprises several major cellular components. The availability of messenger RNA (mRNA), transfer RNA (tRNA) and ribosomal RNA (rRNA), as well as their initiation and elongation, are important in determining the rate of synthesis. At the transcriptional level, the concentration of mRNAs for particular proteins is determined by the myonuclear and/or the mitochondrial density and the transcription factors required for promoter activity. While at the translational level, the number of ribosomes (i.e., rRNA) and the availability and the state of activation of initiation and elongation factors control the rate of translation of mRNA into protein (Bolster et al. 2003).
Myonuclei and satellite cells
Since muscle fibers are multinucleated cells, each myonucleus controls transcription and consequent protein synthesis of a limited amount of cytoplasm. In mammalian as well as amphibian muscle fibers, the number of myonuclei is positively related to the cross-sectional area of the fibers (Allen et al. 1995; Jaspers et al. 2006). Comparison of myonuclear numbers in high and low oxidative fibers has shown that high oxidative fibers contain more myonuclei per mm fiber length, per cross-sectional area and consequently per volume cytoplasm (Burleigh 1977; Schmalbruch and Hellhammer 1977; Tseng et al. 1994). In mammals, the myonuclear density is around 40 per nanoliter cytoplasm in high oxidative fibers and around 20 per nanoliter in low oxidative fibers (Tseng et al. 1994). In contrast, amphibian muscle shows about two to three times lower myonuclear density (Jaspers et al. 2006), which corresponds well with differences in physiological temperature and a protein turnover rate that is about threefold higher in mammalian muscle (Sayegh and Lajtha 1989). Several studies have demonstrated that muscle hypertrophy is associated with, and dependent on, the addition of newly formed myonuclei, whereas muscle atrophy and disease appear to be associated with loss of myonuclei. Together, these data suggest that the myonuclear domain is differentially regulated in high versus low oxidative fibers and that the number of myonuclei per fiber may be important in maintaining size-related differences between fibers.
Based on the idea that each nucleus can only supply a limited amount of cytoplasm with the necessary gene transcripts, addition of myonuclei seems a prerequisite for muscle fiber hypertrophy. As myonuclei lack the ability of mitosis, nuclear accretion stems from a pool of normally quiescent satellite cells that can be induced to differentiate and proliferate and subsequently to fuse with existing myofibers. Although the debate regarding the requirement of satellite cells for myonuclear accretion is ongoing, there are indications that hypertrophy of rat and human muscle fibers by more than 25% is accompanied by the addition of myonuclei (McCarthy and Esser 2007a; O’Connor and Pavlath 2007; Petrella et al. 2006). This supports the necessary role of myonuclei in the induction of hypertrophy. In the heart, the number of nuclei does not increase during hypertrophy. The limited adaptability of cardiomyocytes to overload may partially underlie the development of chronic heart failure occurring at 14 nuclei per nanoliter in rats (Des Tombe et al. 2002). During postnatal development, growth of the satellite cell population in skeletal muscle is associated with an increased rate of nuclear accretion and appears to be twofold higher in high than in low oxidative fibers (Lagord et al. 1998; Moss and Leblond 1971). In adult rats, in the predominantly high oxidative soleus muscle, 12% of the myonuclei belong to satellite cells, whereas in the low oxidative extensor digitorum longus muscle (EDL), the fraction is only 4% (Schultz et al. 2006). In summary, in various vertebrate species, the high oxidative skeletal muscle fibers show higher densities of myonuclei and larger populations of satellite cells compared to low oxidative fibers. Moreover, the rate of nuclear accretion seems higher in high oxidative fibers. Such higher myonuclear density indicates that the high oxidative fibers may have a relatively larger potential for transcription.
Mitochondrial DNA
Mammalian mitochondrial DNA (mtDNA) contains a limited number of genes, which include those encoding 13 polypeptides that are essential for energy production via oxidative phosphorylation, and 22 tRNA and 2 rRNA genes. The mtDNA can replicate independently of nuclear DNA (nDNA), but as in muscle cells the nDNA controls the expression of all other mitochondrial and myofibrillar proteins, and mitochondrial synthesis requires the cooperation of both the nuclear and mitochondrial genomes (Hood 2001).
Few studies are available on the physiological regulation of mitochondrial protein synthesis skeletal muscle and in myocardium (Hock and Kralli 2009). In mammalian striated muscle, the concentrations of mtDNA, mtmRNA and mtrRNA all vary in direct proportion to changes in oxidative capacity. This, combined with the observation that mitochondrial density is also proportional to the oxidative capacity, both in vitro and in vivo, indicates that the expression of mitochondrial genes in striated muscle is proportional to their copy number (Hoppeler and Billeter 1991; van der Laarse et al. 1989; Williams 1986). Based on these findings, it can be concluded that both myonuclear and mitochondrial density are important for maintaining synthesis of proteins associated with oxidative metabolism. Since high oxidative fibers show higher densities of myonuclei and mitochondria, and mitochondrial biogenesis requires both mitochondrial and nuclear DNA, their capacity for mitochondrial protein synthesis is higher compared to the low oxidative fibers.
Transcription
Transcriptional control of myofibrillar protein primarily depends on the abundance and kinetic properties of RNA polymerases, RNAses and the role of transcriptional co-factors that modulate availability of RNA for translation. Together, these properties determine the amount of available mRNA of specific genes in the cell.
It is currently unknown whether transcription rates of RNA polymerases or RNAses differ between fiber types. Biochemically measured transcription rates are in the range of 1.0–4.3 kilobases min−1 and have been suggested to be gene dependent (Darzacq et al. 2007). In human muscle tissue homogenates, an abundance of type I MyHC mRNA, compared to total MyHC mRNA, is associated with high protein synthesis rates, whereas abundance of type IIA MyHC mRNA is associated with low protein synthesis rates (Toth and Tchernof 2006). Several studies showed that the rate of amino acid uptake was two- to threefold higher in high than in low oxidative muscles (Goldberg 1967; Hood and Terjung 1987). Quantitative analysis of RNA gene-bound and unbound fractions of RNA polymerase II showed that ~75% of the polymerases were free and diffusing, whereas the remaining 25% were immobile and suggested to be engaged in transcription (Kimura et al. 2002). As only a quarter of the polymerase capacity is engaged in transcription at a given time, this may suggest that the size of the RNA polymerase pool does not limit transcriptional capacity.
Quantifying mRNA levels of structural muscle protein in different fiber types requires accurate normalization, which is usually done relative to housekeeping genes necessary for basic cellular function (e.g., β-actin, GAPDH and 18S rRNA). Apparently, expression levels of these housekeeping genes can vary depending on the experimental model, species, pathological condition (e.g., Bas et al. 2004; Plomgaard et al. 2006) and the type of muscle (Fig. 2; see supplementary section I for methods). To our knowledge, very little comparative data are available regarding gene transcription in high and low oxidative muscle fibers. Using in situ hybridizations, it has been shown in rat muscle fibers that the amount of MyHC mRNA per microgram total RNA did not differ between fiber types (Habets et al. 1999). However, the total RNA content in high oxidative type I fibers was twofold higher compared to IIA fibers and five- to sixfold higher than IIB fibers with the lowest oxidative capacity. As a consequence, the MyHC mRNA content was substantially higher in high compared to low oxidative fibers. Thus far, fiber type-specific α-skeletal actin levels have not been reported. Using a quantitative PCR technique, we have quantified that predominantly high and low oxidative muscles contain different levels of 18S rRNA and α-skeletal actin mRNA (Fig. 2). Total RNA per microgram muscle tissue is 2.3-fold higher in the high oxidative soleus compared to the low oxidative EDL (Fig. 2a). The fraction of 18S rRNA and α-skeletal actin mRNA on the total RNA is similar in both muscles (Fig. 2b). However, taking into account normalization per microgram muscle tissue, the 18S rRNA and α-actin mRNA levels are 2.1 and 2.3-fold higher, respectively, in soleus compared to EDL (Fig. 2c). Within Xenopus iliofibularis muscles, we found positive correlations (r 2 = 0.71 and r 2 = 0.54, p < 0.0001) between mitochondrial density and α-actin expression (Suppl. Fig. 1, see supplementary section II for methods), which also indicates that within a muscle high oxidative muscle fibers contain higher α-actin expression compared to low oxidative fibers.
Differences in mRNA concentrations of glyceralde-3 phosphate dehydrogenase (GAPDH), 18S RNA, α-skeletal actin (α-sk actin), muscle ring finger-1 (MuRF1) and muscle atrophy F-box (MAFbx) in high oxidative rat soleus (SO) and low oxidative extensor digitorum longus (EDL) muscles (n = 6) (for methods see supplementary section I). a Total RNA (μg) normalized to muscle tissue weight (mg) showed that SO contains 2.3-fold more total RNA per milligram muscle tissue compared to EDL (p < 0.001). b mRNA normalized to total RNA relative to EDL. No differences between SO and EDL were found for any marker, except for GAPDH that showed 2.7-fold lower expression in SO (p < 0.001). c mRNA normalized to muscle tissue weight relative to EDL. GAPDH showed no significant difference between SO and EDL, whereas 18S RNA (2.1-fold), α-sk actin (2.3-fold), MuRF1 (2.1-fold) and MAFbx (1.8-fold) were all higher in SO (p < 0.05). Asterisks indicate significant difference compared to EDL. Systematic comparison of oxidative capacity and fiber cross-sectional area (CSA) from various hind limb muscles in the rat (Armstrong and Phelps 1984) show that SO predominantly (~90%) consists of slow contracting fibers with high oxidative capacity, whereas EDL contains largely (~60%) fast contracting glycolytic fibers with the lowest oxidative capacity. Within EDL, the high oxidative fibers show significantly smaller CSA than low oxidative fibers and also high oxidative fibers in SO show smaller CSA compared to the low oxidative fibers in EDL (Armstrong and Phelps 1984; Nakatani et al. 1999). Based on these observations and the data from Figs. 1 and 2, it can be concluded that high oxidative fibers are generally smaller and also contain more 18S RNA, α-sk actin-, MuRF1- and MAFbx-mRNA, compared to low oxidative fibers. The literature on rat SO and EDL fiber type composition does not unambiguously show that high oxidative fibers have smaller CSA compared to low oxidative fibers (Deveci et al. 2001; Kupa et al. 1995; Torrejais et al. 1999). The inconsistencies in CSA data of the latter studies compared to other studies with a more systematic approach (Armstrong and Phelps 1984; Nakatani et al. 1999) may be related to age, gender, muscle region or the effect of treatment. In addition, the differences in CSA between high and low oxidative fibers were not always tested for statistical significance. This impairs comparison of these studies, largely because classification of the muscle fiber type highly depends on the reaction intensity of the staining in different fiber cross sections and therefore may yield considerable variation in the estimation of fiber populations
The 2.3-fold higher total RNA content in the high oxidative rat soleus compared to the low oxidative EDL (Fig. 2a) corresponds well with the study of Habets et al. (1999), which showed two to sixfold higher total RNA content in high compared to low oxidative fibers. It appears that high oxidative fibers contain substantially more total RNA, higher MyHC, α-actin mRNA as well as about twofold higher myonuclear densities compared to low oxidative fibers. Based on these observations, it may be hypothesized that the myonuclear density largely contributes to the availability of mRNA in high and low oxidative fibers. Further analysis needs to be done to test this hypothesis.
Translation
About 80–85% of the cellular RNA is ribosomal, and in human and rat heart muscle it has been shown that the rRNA content reflects the number of ribosomes (Millward et al. 1973; Razeghi et al. 2006). Assuming that the stability of mRNA, ribosomes and polysomes are similar in high and low oxidative fibers, the higher total RNA content in the rat high oxidative soleus muscle compared to the low oxidative EDL (Fig. 2a; Habets et al. 1999) implies that high oxidative fibers have a higher capacity to synthesize protein.
In addition to the amount of ribosomes, the availability and state of activation (i.e., state of phosphorylation) of initiation and elongation factors and their binding proteins can also be differentially regulated in fiber types. Habets et al. (1999) showed that eukaryotic elongation factor (eEF)-1α expression was higher in high oxidative fibers. For other factors affecting the rate of translation initiation and elongation, only indirect information is available from experiments studying the fiber type-specific expression and activation of these factors in response to various exercise protocols (see “Mechanical loading”).
Co-factors affecting protein synthesis
Transcription rate and stability of mRNA are also regulated by various co-factors that either enhance or inhibit the expression of muscle genes. For example, recently discovered microRNAs (miRNAs) negatively regulate protein synthesis by targeting mRNA for degradation thereby inhibiting transcription, or by blocking mRNA binding sites and subsequently inhibiting translation (Bartel 2004). Thus far, four skeletal muscle-specific miRNAs (miRNA-1, -133, -206, and -208) have been shown to be involved in proliferation and differentiation of myoblasts (Chen et al. 2006), cardiac function and hypertrophy (Sempere et al. 2004). Differential expression in low and high oxidative muscles was found for miRNA-206, which was sevenfold higher in the high oxidative soleus compared to the low oxidative plantaris, whereas expression levels of other miRNAs were similar in these muscles (McCarthy and Esser 2007b). These results indicate that miRNAs can be differentially regulated in high and low oxidative fibers. Whether miRNA-206 and other miRNAs indeed have a fiber type-specific function and whether they have a role in regulating mRNA levels during fiber size adaptation require further investigation.
Fiber type–fiber size paradox
Taken together, high oxidative fibers have a larger potential for transcription (i.e., satellite cells, myonuclei, mitochondria and mRNA) compared to low oxidative fibers. Also, these fibers show a relatively large total ribosomal protein content. This indicates that high oxidative fibers possess a larger capacity for protein synthesis, which seems paradoxical since these fibers remain relatively small. However, this apparent paradox may not be a quantitative one, as there is a discrepancy in mass of large myofibrillar proteins and much smaller oxidative proteins. On the other hand, several factors involved in translation initiation and elongation are differentially expressed and activated in high and low oxidative fibers. This could suggest that one or more of these components of the synthesis machinery are less responsive to contractile activity in high oxidative fibers, thereby reducing translational efficiency and limiting the capacity to synthesize myofibrillar protein. Alternatively, if translational efficiency or the function of other components of the synthesis machinery is not impaired, then the protein synthesis rate is likely to be balanced by a high rate of protein degradation resulting in a higher turnover rate in the high oxidative fibers. The latter would imply a larger capacity of the machinery for protein degradation in high oxidative fibers.
Fiber type-related differences in machinery for protein degradation
The rate of protein degradation in skeletal muscle is largely controlled by oxidative stress and three proteolytic enzymatic pathways: (1) the lysosomal system; (2) calcium-mediated proteases such as caspases and calpains; (3) the ubiquitin–proteasome system (Powers et al. 2007). In addition, expression and activation of several co-factors lead to enhanced or decreased activity of the catalytic enzymes. The susceptibility of proteins to degradation may also depend on conformational stability of the protein, which is determined by factors such as the intracellular temperature, reactive oxygen species, cellular energy status and pH. Modulation of protein degradation rate affects the rate of protein turnover and largely determines whether contractile protein is gained or lost. As a consequence, an increase in the rate of catabolism at a constant rate of protein synthesis results in muscle atrophy.
Lysosomal proteolysis
During muscle atrophy, lysosomal cathepsins have been suggested to be involved in initial breakdown of sarcolemmal proteins such as channels and receptors. Subsequent ubiquitination of these proteins make them target for lysosomal systems, thereby reducing their contribution to protein synthesis. In vitro, cathepsins appear responsible for breaking down myofibrillar protein (Dufour et al. 1989; Mayer 2000). Although assessing the role of lysosomal proteolysis is strenuous as it requires massive inhibition of all types of lysosomal proteases, increased activities of cathepsin have been shown in soleus and extensor digitorum muscles from hind limb-suspended rats with higher concentrations being found in the soleus (Goldspink et al. 1986). Others have also reported that high oxidative fibers of rat contain higher levels of cathepsin than low oxidative fibers suggesting a higher potential for degradation in these fibers (Kominami et al. 1985). These results are consistent with the observation that cathepsins are present in high levels in tissues with high protein turnover (Bechet et al. 2005).
Calcium-dependent proteolysis
Ca2+-dependent proteases such as calpains and caspases are involved in breaking down cytoskeletal and myofibrillar proteins. Calpain isoforms (e.g., μ-calpain and m-calpain) can be activated by different concentrations of cytoplasmic calcium and are mainly known for cleaving cytoskeletal proteins that anchor the myofibrillar proteins, such as nebulin and titin (Goll et al. 2003). Many caspases have been identified of which several are important in muscle degradation (see for review Powers et al. 2007). In skeletal muscle, caspases are particularly involved in myonuclear apoptosis and degradation of the actin–myosin complex. During conditions of disuse, calpains and caspases are up-regulated and inhibition of calpains results in attenuation of the atrophy. As a result of the activity of caspases and calpains, monomeric actin and myosin proteins become available for degradation by the proteasome (Du et al. 2004; Taillandier et al. 1996) (see below).
It is unknown whether a fiber type-specific response in calcium-mediated proteolysis exists. However, intracellular calcium concentrations [Ca2+]i are generally high in cardiac myocytes and high oxidative skeletal muscle fibers (Batkai et al. 1999; Dibb et al. 2007). As high [Ca2+]i are associated with increased calpain and caspase activity, we hypothesize higher activities of caspases and calpains in high compared to low oxidative fibers, which would imply a higher rate of cytoskeletal protein degradation in these high oxidative myocytes.
Proteasome-mediated proteolysis
The ubiquitin–proteasome system is responsible for breakdown of the majority of myofibrillar proteins and is both ATP and ubiquitin dependent (Taillandier et al. 1996). In the process of ubiquitination, proteins are marked for degradation by three classes of enzymes, known as E1 (activating), E2 (conjugating) and E3 (ligating), after which the proteasome degrades the ubiquitin substrates. During various conditions of disuse, such as denervation, unloading, hind limb suspension and fasting, the expression of two muscle-specific ubiquitin-ligases, muscle atrophy F-box (MAFbx/atrogin-1) and muscle ring finger (MuRF), was found to be up-regulated. Inhibition of either one resulted in attenuation of atrophy (Bodine et al. 2001a). MAFbx and MuRF are controlled by the Forkhead box transcription factors O (FOXO) subfamily of transcription factors and the nuclear factor kappa-B (NF-κB) (Nordquist et al. 2007; Sandri et al. 2004). MAFbx and MuRF contribute to atrophy by up-regulation of proteasome components and by inhibition of transcription of slow-type structural muscle genes and translation (see also ‘FOXO–E3 ligase–proteasome pathway’ and ‘NF-κB pathway’).
To our knowledge, there are no experimental data available on fiber type-specific expression of ubiquitin-ligases or proteasome components. We quantified both MAFbx and MuRF mRNA expression relative to total RNA and found no difference between high oxidative soleus and low oxidative EDL (Fig. 2b). However, as the soleus contains 2.3-fold more total RNA per muscle tissue weight (Fig. 2a), expression levels of MAFbx and MuRF are significantly higher (1.8- and 2.1-fold, respectively) compared to EDL (Fig. 2c). In contrast, FOXO1 mRNA expression was found to be lower in high oxidative mouse soleus compared to the low oxidative gastrocnemius, tibialis anterior and quadriceps muscles (Allen and Unterman 2007). In vivo, FOXO1 inhibits expression of high oxidative fiber-related genes and the function of oxidative metabolism-enhancing factors (Kamei et al. 2004). In addition, skeletal muscles of FOXO1 over-expressing mice had fewer type I fibers as well as smaller type I and type II fibers (all isoforms) (Kamei et al. 2004).
Oxidative stress-mediated proteolysis
Classical in vitro experiments have shown that 2–5% of total oxygen becomes a superoxide or free radical (Boveris and Chance 1973). This may overestimate in vivo rates of oxidant production as assessment of ROS production from different sites in mitochondria indicated that upper estimates of proportion of the electron flow giving rise to superoxide was only 0.15% (St-Pierre et al. 2002). Despite the controversy on estimating the proportion of free radicals in vivo (Jackson et al. 2007; Murphy 2009), mitochondria can be considered the major source of free radicals since electron transport accounts for ~85% of the oxygen consumed by the cell (Challoner 1968). Under normal physiological conditions (i.e., normal PO2 levels), a variety of reactive oxygen species (ROS) and reactive nitrogen species (RNS) are produced in skeletal muscle fibers, which play a critical role in the adaptation of muscle (for review see Jackson et al. 2007; Powers et al. 2007). At normal PO2 levels, ROS promote adaptation and survival in many cell types. However, under conditions of oxidative stress (i.e., hypoxia or hyperoxia), ROS production increases and induces myonuclear apoptosis in vivo and in vitro (Duranteau et al. 1998). High levels of ROS (e.g., as a result of exercise or reperfusion injury) activate caspases via Ca2+ release from the sarcoplasmatic reticulum and also induce mitochondrial permeability transition followed by cytochrome c release, which leads to myonuclear apoptosis and myofibrillar degeneration (Garrido et al. 2006; Primeau et al. 2002). In addition, ROS are also involved in regulating Ca2+-meditated proteolysis (through calpain activation) and increased proteasome activity through NF-κB and the E3 ligases (Powers et al. 2007).
In relation to exercise, many studies have shown elevated ROS production, which is assumed to be associated with the increased oxygen consumption that occurs with elevated mitochondrial activity (Herrero and Barja 1997; Malinska et al. 2009; Powers et al. 1999). However, exercise also induces antioxidant production and structural changes of the mitochondria, which have been related to a reduction in free radical concentration (Anderson and Neufer 2006; Leeuwenburgh et al. 1997; Molnar et al. 2006). Recently, it has been suggested that the production of ROS and antioxidant scavengers may be tightly regulated by an internal control mechanism, involving uncoupling proteins and antioxidants (Brand et al. 2004). Such a mechanism would keep ROS concentrations in the cell within a range that is beneficial for cellular survival. To our knowledge, measurements on intracellular ROS generation and scavenging in high and low oxidative fibers have not been undertaken, but the available data for muscle cells indicate that contractile activity increases ROS production two to fourfold (McArdle et al. 2005; Vasilaki et al. 2006). As antioxidant production also increases with exercise, this may indicate that ROS and antioxidant scavengers are both higher in high oxidative fibers compared to low oxidative fibers. As yet the relative differences in ROS concentrations between fiber types and their role in regulating fiber size are not clear. To obtain insight into the contribution of ROS in the fiber type-related regulation of size, future experiments need to determine the concentrations of different types of free radicals and their scavengers in high and low oxidative fibers by systematically assessing the numerous endogenous sites and sources that produce ROS and antioxidants.
Pathways and stimuli regulating protein turnover in different fiber types
From the first part of this review, it becomes evident that high oxidative muscle fibers show a larger potential for protein synthesis and that some components of the degradation machinery are also present in higher quantities compared to low oxidative fibers. This suggests that high oxidative fibers have a relatively high rate of protein turnover, which may limit hypertrophy in these fibers. The machinery for protein turnover is regulated by many different pathways and stimuli. Since muscle fibers have limited capacity to hypertrophy and increase oxidative capacity at the same time, this may imply that competition exists between turnover rates of structural muscle protein (i.e., myofibrillar proteins) and protein involved in metabolism (i.e., mitochondrial proteins). Such competition is likely the result of interactions between signaling pathways either involved in synthesis or breakdown of the structural and metabolic proteins. The stimuli regulating these pathways are related to muscle activity (frequency and firing pattern of action potentials, intracellular calcium), mechanical loading, growth factors, vitamins, binding factors and cytokines and factors associated with cellular energy and oxygen levels, such as hypoxia, redox potential and AMP:ATP ratio.
Signaling pathways involved in muscle protein turnover
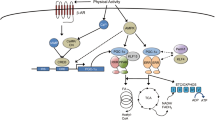
Several signaling pathways are known for their role in regulation of muscle fiber size (Suppl. Fig. 2A–E). These pathways control the rate of protein turnover at the level of transcription (calcium/calmodulin pathways; MAP-kinase pathways), translation (PI3K–Akt–mTOR), degradation (FOXO–E3 ligase–proteasome and NF-κB pathway) or a combination of these (AMPK–PGC-1α). The interactions between the pathways (Fig. 3) determine the rates of protein synthesis and degradation and whether contractile or metabolic protein is gained or lost.
Interactions of signaling pathways and their stimuli involved in turnover of structural muscle (i.e., contractile) protein and protein associated with high oxidative metabolism. In response to contractile activity, calcium increases intracellularly through stretch-activated Ca2+ channels and from calcium stores. In addition, growth factors and cytokines are secreted in the extracellular matrix by the muscle fibers where they can bind receptors and activate signaling pathways. The type of contractile activity or mechanical loading combined with the balance between cellular energy (AMP:ATP) and oxygen levels (i.e., ROS production) determine fiber type-specific activation of pathways and thus whether contractile protein is gained or lost (for details see text). AAs amino acids, AMPK AMP-activated protein kinase, bFGF basic fibroblast growth factor, ECM extracellular matrix, FAK focal adhesion kinase, FOXO Forkhead box transcription factors O subfamily, GF + CK growth factors and cytokines, HGF hepatocyte growth factor, IL interleukin, IGF-I insulin-like growth factor-I, MAFbx muscle atrophy F-box, MAPK mitogen-activated protein kinases, MGF mechano-growth factor, mTOR mammalian target of rapamycin, MuRF muscle ring finger, NF-κB nuclear factor kappa-B, PGC-1α peroxisome proliferator-activated receptor γ coactivator-1α, PI3K phosphatidylinositol-3 kinase, PLD phospholipase D, p70S6K 70-kDa ribosomal protein S6 kinase, ROS reactive oxygen species, SC satellite cells, SR sarcoplasmatic reticulum, SRF serum response factor, TNF-α tumor necrosis factor-α, Vps34 vacuolar protein sorting mutant 34
Calcium/calmodulin pathways
Intracellular calcium [Ca2+]i regulates the activity of calcineurin, a serine/threonine phosphatase, and calcium/calmodulin protein kinase (CaMK), both in a calmodulin-dependent way (Klee et al. 1998; Schulman 1993). Activity of calcineurin is enhanced in skeletal muscle in response to sustained low-amplitude oscillations of [Ca2+]i while it remains insensitive to transient high-amplitude oscillations of [Ca2+]i (Dolmetsch et al. 1997). In contrast, CaMKII and CaMKIV respond to physiological high-amplitude Ca2+ signals, which, in skeletal muscle, correspond to Ca2+ levels achieved at stimulation frequencies higher than 30 Hz (Chin 2005). Downstream targets of calcineurin and CaMK (Suppl. Fig. 2A) are myogenic transcription factors, such as nuclear factor of activated T cells (NFAT), myocyte enhancer factor-2 (MEF-2) and serum response factor (SRF) (Davis et al. 2003; Dolmetsch et al. 1997; Wu et al. 2001). Activation of calcineurin and CaMK causes conformational changes of these transcription factors, which either activate them or stimulate translocation from cytoplasm to the nucleus.
Calcineurin has been attributed a fiber type-specific role in inducing fast-to-slow transitions in MyHC isoforms (Chin et al. 1998). In addition, calcineurin regulates a large subset of genes involved in oxidative metabolism (for instance myoglobin) and increased glucose uptake and fatty acid oxidation (Bigard et al. 2000; Chin 2005) (Suppl. Fig. 2A). Both calcineurin and CaMK can increase mitochondrial biogenesis through increased expression of peroxisome proliferator-activated receptor γ co-activator 1α (PGC-1α) (Lin et al. 2002; Wu et al. 2002) (see ‘AMPK–PGC-1α’). However, controversy exists on the role of calcineurin and CaMK in regulating fiber size.
Calcineurin appears to be involved in myonuclear accretion in high, but not in low oxidative fibers (Mitchell et al. 2002). The hypertrophic effects of calcineurin are also indicated by the hypertrophic response of myoblasts in culture while exposed to activated calcineurin (Musaro et al. 1999; Semsarian et al. 1999). Furthermore, inhibition of calcineurin activity during mechanical overload, recovery from disuse atrophy or in muscles from transgenic mice over-expressing insulin-like growth factor-I (IGF-I) showed blocking of the hypertrophic response (Dunn et al. 1999; Mitchell et al. 2002; Musaro et al. 1999; Semsarian et al. 1999). In contrast, other studies using various models of hypertrophy did not show an active role for calcineurin in increasing fiber size in muscles of mice and rats (Bodine et al. 2001b; Musaro et al. 2001; Serrano et al. 2001). Furthermore, mice over-expressing calcineurin showed no evidence for hypertrophy (Naya et al. 2000). Altogether, it appears that calcium/calmodulin-activated pathways are particularly involved in the synthesis of slow-type gene expression and oxidative metabolism. In addition, although activation of calcineurin alone is not sufficient for inducing hypertrophy, it may do so, for example, in combination with other essential pathways mediated by IGF-I (see growth factors).
MAPKinase pathways
The three best characterized subfamilies of mitogen-activated protein kinase (MAPK) signaling are (1) c-Jun N-terminal kinase (JNK); (2) extracellular regulated kinase (ERK); and (3) the 38 kDa stress-activated protein kinase (p38). Activation of MAPK is mediated by a set of three sequential protein kinases that are generally induced by: (1) receptor binding of stimuli (e.g., IGF-I); 2) protein kinase C activation; or (3) by integrin or dystroglycan signaling (Carson and Wei 2000; Florini et al. 1996; Rando 2001; Ruwhof and van der Laarse 2000). Activated MAPK will phosphorylate and activate myogenic and metabolic transcription factors, as well as other kinases that affect downstream signal transduction (Suppl. Fig. 2B). The fiber type-specific functions of ERK, JNK and p38 in muscle are briefly summarized.
The ERK pathway is involved in regulation of protein synthesis, particularly in the induction of slow fiber phenotypes and the up-regulation of genes involved in glucose transport, glyconeogenesis and angiogenesis (Koulmann and Bigard 2006; Murgia et al. 2000). Furthermore, ERK has been shown to reduce proteasome activity, mitochondrial breakdown and myonuclear apoptosis, thereby promoting cellular survival (Powers et al. 2007). In addition, ERK has also been attributed a role in stretch-induced cardiac hypertrophy by increasing c-fos and α-actin expression (Sadoshima and Izumo 1993; Yazaki and Komuro 1992). Although very little data are available on fiber type-specific MAPK expression and/or activity, ERK-1 and ERK-2 concentrations are significantly different in rat soleus and EDL muscles, with more ERK being present in the high oxidative soleus (Atherton et al. 2004; Wretman et al. 2000). This might suggest a fiber type-specific role for ERK. On the other hand, ERK is also critically related to IGF-I-induced hypertrophy in rat plantaris (Haddad and Adams 2004), which is predominantly composed of type II fibers (type IIA: ~40%, type IIB: ~40%; (Armstrong and Phelps 1984). This might also suggest a role for ERK in fiber type-related regulation of size.
Activation of p38 promotes PGC-1α expression and subsequent mitochondrial biogenesis (Akimoto et al. 2005) (Suppl. Fig. 2B). Other downstream targets of p38 are MEF-2, which is involved in slow-type gene expression, and p53 that mediates myonuclear apoptosis (Powers et al. 2007). The p38 MAPK pathway also plays a role in protein degradation by promoting MAFbx expression and activation of NF-κB (Li et al. 2005; Powers et al. 2007; Zhao et al. 1999).
Among the specific targets of JNK are c-jun, c-myc and ATF-2, which are involved in the expression of structural muscle protein (Clerk and Sugden 1997) (Suppl. Fig. 2B). JNK is also active during oxidative stress and mediates myonuclear apoptosis and mitochondrial breakdown (Powers et al. 2007). To our knowledge, there are no data available on fiber type-specific expression of p38 and JNK except in response to various activity protocols (see “Mechanical loading”).
MAPKs differ in their interaction with transcription factors, providing a tool for selectively targeting transcription factors. Although the function of p38 in muscle remains ill defined, the current literature indicates that p38 is involved in high oxidative gene expression and also in proteasome-mediated degradation. JNK induces gene expression of structural muscle proteins and promotes myonuclear apoptosis, whereas ERK mainly has a protective role in promoting cellular survival and stimulating the oxidative metabolism. However, as each MAPK shares targets and functions with many upstream and downstream kinases and transcription factors, specificity of the pathways and their fiber type-specific role are not fully resolved.
PI3K–Akt–mTOR
A central pathway involved in hypertrophy is regulated at the translational level by the serine/threonine kinase Akt (or PKB). In muscle, Akt is activated by the upstream phosphatidylinositol 3-kinase (PI3K), either induced by receptor binding or by integrin-mediated activation of focal adhesion kinase (FAK), such as in cardiac myocytes (Franchini et al. 2000; Sakamoto et al. 2002) (Fig. 3). PI3K activates Akt, which then has the ability to phosphorylate and change the activity of many signaling molecules. Among these are the mammalian target of rapamycin (mTOR) and glycogen synthase kinase-3β (GSK-3β), which play a crucial role in the regulation of translation (Cross et al. 1995). Akt activates mTOR via phosphorylation and inactivation of tuberous sclerosis complex-2 (TSC-2) (Manning et al. 2002; Potter et al. 2002). Subsequently, mTOR phosphorylates and activates the 70-kDa ribosomal protein S6 kinase (p70S6K), which results in increased translation either directly or indirectly by activating initiation and elongation factors, eIF-2, eIF-4E (through 4E-BP) and eEF-2 (Bodine et al. 2001b; Hornberger et al. 2001) (Suppl. Fig. 2C). In addition, Akt also phosphorylates and inactivates GSK-3β, thereby activating translation via initiation factor eIF-2B (Jefferson et al. 1999). Other functions of Akt comprise the negative regulation of protein degradation by inhibiting FOXO-mediated proteasome activity (Stitt et al. 2004) (see ‘FOXO–E3 ligase–proteasome pathway’). However, Akt has also been associated with up-regulation of the proteasome through activation of NF-κB (see NF-κB pathway) in a PI3K-dependent process (Russell et al. 2008). In addition, Akt functions as negative regulator of the oxidative metabolism by inhibiting AMP-activated protein kinase (AMPK) (Hahn-Windgassen et al. 2005) (see ‘AMPK–PGC-1α’).
Although it is clear that the PI3K–Akt–mTOR pathway plays a crucial role in regulating hypertrophy, the fiber type-specific role of this pathway remains unclear. Akt appears to be differentially regulated in high and low oxidative muscles, as it has been shown that both total and phosphorylated Akt concentrations were substantially higher in rat soleus compared to EDL (Sakamoto et al. 2002). On the other hand, also in rat EDL muscle, it was shown that total p70S6K was sixfold higher than in the soleus (Atherton et al. 2004), but the fraction of the phosphorylated protein was substantially lower in EDL compared to the soleus (27 vs. 64%) (Hornberger et al. 2001). Currently, it is unknown whether high and low oxidative fibers contain similar absolute concentrations of phosphorylated p70S6K; however, it appears that that the low oxidative fibers have a larger potential for increasing the phosphorylation state (i.e., activation) of p70S6K and subsequent translation compared to high oxidative fibers.
FOXO–E3 ligase–proteasome
During various conditions of disuse, FOXO transcription factors up-regulate the expression of ubiquitin-ligases (MAFbx and MuRF), which are associated with increased proteasome activity and protein degradation (Lecker et al. 2004; Sandri et al. 2004; Stitt et al. 2004) (Suppl. Fig. 2D). As mentioned above, in response to hypertrophic stimuli, Akt inhibits protein degradation by phosphorylating FOXO, which results in nuclear exclusion of FOXO and a decreased transcription of FOXO target genes (Sandri et al. 2004). However, during muscle atrophy, MAFbx is up-regulated and prevents the Akt-dependent phosphorylation of FOXO (Schisler 2008). Consequently, FOXO remains located in the nucleus causing enhanced expression of MAFbx and MuRF. The expression of MAFbx further increases in a feed-forward mechanism with FOXO, thereby stimulating protein degradation. In addition, FOXO and ubiquitin-ligases also negatively affect protein synthesis. For example, FOXO has been shown to inhibit mTOR signaling, whereas MAFbx targets eIF-3 for degradation (Suppl. Fig. 2D) thereby attenuating protein translation (Lecker et al. 2004; Southgate et al. 2007). At the transcriptional level, FOXO reduces the expression of calcineurin and transcription factors MEF-2 and NFAT, which may attenuate slow-type gene expression (Kamei et al. 2004; Ni et al. 2006) (Suppl. Fig. 2A, 2D).
FOXO1 expression, normalized to β-actin, in low oxidative mouse muscles is higher compared to the high oxidative soleus muscle (Allen and Unterman 2007). Based on the above-mentioned feed-forward mechanism between FOXO and MAFbx, it can be hypothesized that protein degradation is higher in the low oxidative fibers. However, our results show that both MAFbx and MuRF expression was about twofold higher in high oxidative fibers (Fig. 2c). In addition, it appears that FOXO is not involved in atrophy per se, as FOXO1 and FOXO3a proteins are unaltered in human vastus lateralis after 20 days of unilateral limb suspension (Sakuma et al. 2009). These results suggest that an alternative route besides FOXO exists that is capable of inducing the expression of E3 ligases, MAFbx and MuRF. Altogether, FOXO-mediated E3 ligase-proteasome activity appears to play an important role in reducing the fiber size during disuse, both by increasing the rate of protein degradation and by attenuating the rate of protein synthesis. However, it remains to be investigated in what way FOXO expression changes in response to various training stimuli and whether FOXO is also capable of limiting fiber size in response to hypertrophic stimuli.
NF-κB pathway
The NF-κB family of transcription factors is also involved in proteolysis during muscle atrophy. Specific NF-κB members have been related to atrophy and cachexia in response to disuse and chronic diseases (Jackman and Kandarian 2004). NF-κB is an important transcription factor in disuse atrophy and associated with increased expression of E2 and E3-ligases in mice leg muscles (Cai et al. 2004). However, whether NF-κB directly or indirectly affects the E3-ligases has not been elucidated yet. It is clear that NF-κB induces atrophy (Suppl. Fig. 2D, Fig. 3), but whether this is FOXO dependent also remains to be determined.
In rats, overall nuclear NF-κB (p65) expression was found to be 56% higher in high oxidative soleus compared to the low oxidative superficial vastus lateralis (Phillips and Leeuwenburgh 2005). NF-κB levels in rat soleus muscle were also threefold higher compared to those in EDL (Atherton et al. 2004). In addition, NF-κB showed a tenfold higher activation in soleus of rat after 7 days of unloading, whereas in EDL no change was detected (Hunter et al. 2002). These results indicate a differential expression of NF-κB between fiber types, suggesting that NF-κB-induced E3 ligase-proteasome activity is higher in high oxidative fibers. In addition, high levels of NF-κB signaling have also been associated with reduced myonuclear apoptosis in mice and rats (Phillips and Leeuwenburgh 2005). This effect could be related to the NF-κB-induced inhibition of anti-apoptotic genes (e.g. bcl-2) (Perkins 2000). Altogether, NF-κB appears to play a role in inducing E3 ligase-proteasome activity, particularly in high oxidative fibers and is also involved in regulating myonuclear apoptosis.
AMPK–PGC-1α
AMPK is a highly conserved protein kinase that plays a role in the regulation of many cellular processes. In mammalian adult muscle tissue, AMPK function includes induction of glucose transport, glycogen metabolism, fatty acid oxidation and transcriptional regulation of structural muscle genes (Hardie and Sakamoto 2006).
AMPK controls several processes via the transcriptional co-activator PGC-1α (Suppl. Fig. 2E). PGC-1α, and also functionally similar PGC-1β, are involved in inducing mitochondrial biogenesis and slow-type gene expression via MEF-2 and NFAT (Arany et al. 2007; Garnier et al. 2005; Lelliott et al. 2006; Lin et al. 2002). The regulation of high oxidative gene expression by PGC-1 is mediated by interactions with different isoforms of a family of nuclear hormone receptors, the peroxisome proliferator-activated receptors (PPARs) (Gilde and Van Bilsen 2003). AMPK induces the expression of PGC-1α as well as the activation and expression of PPAR isoforms (Lee et al. 2006; Narkar et al. 2008). AMPK expression appears to be similar in high and low oxidative fibers, while basal AMPK phosphorylation is higher in type IIA fibers compared to type I and IIB fibers (Lee-Young et al. 2009). Concentrations of PGC-1α and PPAR isoforms (β and δ) appear to be higher in high oxidative fibers suggesting that the AMPK–PGC-1α pathway induces a fiber type-specific increase in genes associated with high oxidative metabolism (Lin et al. 2002; Narkar et al. 2008).
Another role of AMPK comprises the inhibition of protein synthesis. It has been well established that AMPK activation suppresses the translation of myofibrillar protein by inhibiting the mTOR–p70S6K pathway via activation of TSC-2 and by phosphorylation and inactivating eEF-2 (Chan and Dyck 2005; Inoki et al. 2003; Mounier et al. 2009). This interaction indicates that AMPK, in addition to being a stimulus of biosynthesis of mitochondria, is an inhibitor of protein synthesis.
Recently, AMPK has also been linked to protein degradation since AMPK activation increases the expression of E3 ligases, MAFbx and MuRF (Krawiec et al. 2007). Several studies have shown that AMPK also activates NF-κB signaling in vitro (Jung et al. 2004; Okayasu et al. 2008). Although AMPK activation also has been related to suppression of NF-κB, presumably through reduced cytokine expression (Li et al. 2007), there are multiple NF-κB family members that are involved in muscle wasting (Hunter et al. 2002). Therefore, the AMPK-induced NF-κB activation may indicate that AMPK signaling increases protein degradation. AMPK may also induce protein degradation through an interaction between PGC-1α and FOXO-mediated protein degradation. Kamei et al. (2004) hypothesized that FOXO inhibits PGC-1α function by binding PGC-1α. However, high expression levels of PGC-1α have also been associated with decreased MAFbx and MuRF protein levels (Sáinz et al. 2009). In addition, Sandri et al. (2006) also showed reasonable data to suggest that PGC-1α inhibits FOXO function. The latter studies raise serious concerns about the role of PGC-1α in protein degradation. Whether AMPK executes its different roles simultaneously in response to its activation remains to be determined, but there are indications that AMPK reduces cell differentiation of myoblasts, thereby reducing hypertrophy, without necessarily accelerating protein degradation (Fulco et al. 2008). This would suggest that other signaling components were also capable of inducing NF-kB-mediated protein degradation. Thus, although there appears to be an interaction between AMPK and NF-κB, clearly more research is necessary to clarify the role of the AMPK–PGC-1α pathway in protein degradation.
In conclusion, AMPK stimulates the oxidative metabolism and slow-type gene expression through PGC-1α and simultaneously attenuates synthesis of protein by inhibiting mTOR and the rate of translation of mRNA. As Akt, which activates mTOR, negatively regulates high oxidative gene expression by inactivating AMPK (see ‘PI3K–Akt–mTOR pathway’), competitive interaction exists between signaling pathways regulating structural and metabolic protein turnover (Fig. 3). This competitive interaction has previously led to the hypothesis that Akt–AMPK serves as a ‘switch’ between pathways inducing a high or a low oxidative phenotype, thereby regulating the muscle fiber size (Atheron et al. 2005; Baar 2006). However, the Akt–mTOR and AMPK interactions may be more complex than suggested by a ‘switch’ mechanism. For example, mTOR activation has been shown to increase mitochondrial gene transcription by interaction with PGC-1α (Cunningham et al. 2007). Also, knockdown of mTOR-binding protein Raptor has been shown to down-regulate genes involved in mitochondrial biogenesis (e.g., PGC-1α) and hyperactivation of Akt, suggesting that mTOR is not only required for hypertrophy but also for regulation of metabolic function (Bentzinger et al. 2008).
Stimuli and the status of activity of fiber type-specific signaling pathways
In vivo, multiple signals or stimuli are received by muscles, which trigger one or more of the above-mentioned signaling pathways involved in the regulation of muscle protein turnover. The key stimuli are all related to the type of contractile activity, ranging from sustained, low force activity (endurance training) to short and high force activity (resistance training). Contractile activity and the associated mechanical loading can activate these pathways either directly or indirectly via changes in [Ca2+]i and the expression of growth factors and cytokines. In addition, changes in cellular energy and oxygen levels, which are affected by contractile activity as well, also regulate activation of signaling pathways specific for high and low oxidative fibers.
Mechanical loading
Mechanical loading is associated with the regulation of muscle mass; however, the cellular and molecular mechanisms that contribute to the transduction of mechanical signals into a hypertrophic response are complex and still a matter of ongoing research. The MAPKinase and the Akt–mTOR pathways play important roles in increasing the rate of protein synthesis in response to mechanical loading (Glass 2005; Ruwhof and van der Laarse 2000), particularly by increased expression of growth factors (see below). Recently, other growth factor-independent mechanisms have been proposed that connect mechanical events at the membrane to the intracellular signaling structure, among which are the stretch-activated channels (SACs) and the integrin-focal adhesion complexes (FACs) (Spangenburg 2009). The inhibition of SACs has been shown to result in attenuation of the Akt–mTOR signaling pathway, reduced hypertrophy and a lack of expected functional adaptations in response to loading (Butterfield and Best 2009; Spangenburg and McBride 2006). It has been hypothesized that the SACs allow the influx of ions such as Ca2+, which affects both protein synthesis and degradation (Yeung et al. 2005) (see “Intracellular calcium”).
The integrin-focal adhesion complexes seem to be involved in conversion of mechanical signals into intracellular signals. In endothelial cells, mechanical loading is associated with the recruitment of proteins to focal adhesion sites underlying the extracellular matrix that particularly contain integrins (Wang et al. 1993). In fibroblasts, clustering of integrins is followed by an increase in phosphorylation and recruitment of several other proteins to the FAC, such as FAK and paxillin (Burridge et al. 1992; Kornberg et al. 1992). For muscle tissue, the precise mechanism that links FAK to the integrins is still unclear, although it has been shown that FAK activation and the expression of FAK and paxillin are increased in response to mechanical loading (Fluck et al. 1999). FAK has also been associated with increased translational signaling, either by inducing Akt–mTOR signaling through PI3K (Xia et al. 2004) or by increasing p70S6K activation through mTOR, independently of Akt (Klossner et al. 2009; Xia et al. 2004). In addition, SRF gene expression, which is both mediated through FAK and the MAPKinases, induces both α-actin and IGF-I expression and appears to be related to hypertrophy in response to mechanical loading (Carson et al. 1996; Charvet et al. 2006; Gineitis and Treisman 2001; Miano et al. 2007; Wei et al. 1998). FAK concentration and its phosphorylation level, as well as paxillin, integrin and SRF concentrations appear to be higher in the high oxidative rat soleus compared to the low oxidative plantaris muscle (Gordon et al. 2001). This suggests that mechanical loading can induce a fiber type-specific increase in protein synthesis that involves both FAK and SRF signaling. However, the relative contribution of FAK and PI3K in mechanical loading-induced activation of the Akt–mTOR pathway remains elusive, as recently it has been shown that mechanical loading can activate Akt–mTOR independent of PI3K (Hornberger et al. 2007). In addition, phospholipase D (PLD) and phosphatidic acid (PA) provide another signaling mechanism to mTOR–p70S6K independent of Akt (Fang et al. 2001; Hornberger et al. 2006). Furthermore, the differential hypertrophic effects of various types of mechanical loading via the mTOR–p70S6K pathway may also depend on the nutritional status, as it has been shown that muscle growth is optimized by combining exercise and the ingestion of amino acids and carbohydrates (Deldicque et al. 2005).
MAPKinases are also differentially expressed and activated in high and low oxidative fibers in response to stretch, contractile activity or metabolic stimuli (Csukly et al. 2002; Wretman et al. 2000). p38 expression appears to be higher in high oxidative fibers, whereas the increase in phosphorylation in response to tetanic stimulation is higher in the low oxidative EDL. For ERK and JNK activities, there is no unambiguous information on fiber type-specific expression in response to mechanical loading (Csukly et al. 2002; Wretman et al. 2000). The fiber type-specific role of the various MAPKinases is not clear, mostly because of the lack of information on changes in total and phosphorylated protein in response to mechanical loading. Recently, inhibition of ERK in myotubes had been shown to decrease their size and protein content, while resistance exercise augmented both p38 and ERK1/2 and p70S6K signaling. Furthermore, inhibition of all three MAPK pathways induced NF-κB activity to a greater extent in the rat soleus compared to the gastrocnemius muscle in vivo (Deldicque et al. 2008; Shi et al. 2009). This suggests that MAPK inhibition induces atrophy via NF-κB in high oxidative muscle activity, but it is also likely that inhibition of MAPKs are involved in atrophy in low oxidative muscles via other stimuli and signaling pathways that enhance protein degradation via the proteasome.
Downstream of Akt–mTOR, at the level of translation, the initiation and elongation of translation of mRNA also appears to be regulated by mechanical loading. In rats, increased phosphorylation levels of eIF-2 as well as eIF-4 and its binding protein (4E-BP1) have been shown in response to endurance exercise associated with a high oxidative phenotype, whereas resistance training, denervation or hind limb unloading, inducing a low oxidative phenotype, de-phosphorylates these initiation factors (Atheron et al. 2005; Hornberger et al. 2001; Thomson et al. 2008). In response to an unloading period, concentrations of phosphorylated eEF-2 in rat soleus showed a 50% decrease compared to those in control muscles, while total eEF-2 remained unchanged. In EDL, no effect was found for unloading on phosphorylated or total amount of eEF-2 (Hornberger et al. 2001). These results indicate that there may be a fiber type-specific regulation in amount and activity of the initiation and elongation factors in response to mechanical loading. In addition, after resistance exercise in human vastus lateralis, p70S6K phosphorylation levels are significantly higher in type II fibers (all isoforms) than in high oxidative type I fibers (Koopman et al. 2006). In rat EDL, total p70S6K was sixfold higher compared to the soleus, whereas the fraction of phosphorylated p70S6K was substantially lower in EDL [27% (EDL) vs. 64% (soleus)] (Hornberger et al. 2001). These data suggest that the low oxidative muscle fibers have a larger potential to phosphorylate p70S6K compared to high oxidative fibers, which has been related to increases in translation initiation and subsequent gain in muscle mass (Baar and Esser 1999).
Growth factors
Among the growth factors that have been discovered to play a role in muscle adaptation, the key regulatory factors are the insulin-like growth factor-I (IGF-I Ea and mechano-growth factor, MGF) (Glass 2003; Goldspink 2005) and the transforming growth factor-β (TGF-β) superfamily member, myostatin (Lee and McPherron 2001; Zimmers et al. 2002). IGF-I is a potent stimulator of myofibrillar protein synthesis via the PI3K–Akt–mTOR pathway, the MAPKinases and the calcineurin–PGC-1α pathway (Bodine et al. 2001b; Haddad and Adams 2004; Semsarian et al. 1999) (Fig. 3). In vitro, IGF-I has been shown to activate calcineurin and vice versa, which suggests that IGF-I acts in a feed-forward loop with calcineurin, thereby promoting gene expression associated with high oxidative metabolism (Alfieri et al. 2007; Musaro et al. 1999; Semsarian et al. 1999). Furthermore, IGF-I Ea induces influx of calcium into the cytoplasm via L-type Ca2+ channels (Delbono et al. 1997) (see “Intracellular calcium”) and is involved in recruitment of satellite cells, either independently or in combination with other growth factors such as basic fibroblast growth factor (bFGF) or hepatocyte growth factor (HGF) and MGF (Allen and Boxhorn 1989; Doumit et al. 1993; Yang and Goldspink 2002). In addition, IGF-I also acts as an inhibitor of degradation via inhibition of the FOXO–E3 ligase–proteasome and attenuating caspases-mediated apoptosis (Bodine et al. 2001b; Dalla Libera et al. 2004). These results suggest that IGF-I signaling is important for mediating increases in the capacity for protein accumulation in response to mechanical loading. However, it appears that other routes besides IGF-I/insulin signaling may also contribute to mechanical load-induced hypertrophy, as it has been shown that hypertrophy is possible without a functional insulin-like growth factor receptor (Spangenburg et al. 2008). These and other studies indicate that the debate on whether IGF-I signaling or other mechanical load-induced routes are the major regulators of increasing fiber size has not been settled yet (Stewart et al. 2009).
Myostatin is a negative regulator of muscle size as it stimulates the expression of the FOXO–E3 ligase–proteasome system and inhibits proliferation of satellite cells (McCroskery et al. 2003; McFarlane et al. 2006) (Fig. 3). Myostatin activates FOXO1, while FOXO1 in turn increases myostatin expression, resulting in a feed-forward loop between myostatin and FOXO and a subsequent increase in protein degradation (Allen and Unterman 2007). In addition, FOXO1 also inhibits IGF-1-mediated satellite cell proliferation (Machida and Booth 2004). However, it has been shown that myostatin over-expression in rat tibialis anterior muscle was unable to alter the activity of proteasome components, and treatment of human skeletal muscle cells with myostatin even resulted in a decrease in the expression of E3 ligases (Amirouche et al. 2008; Trendelenburg et al. 2009). Instead, myostatin was suggested to negatively regulate protein synthesis by inhibition of the Akt–mTOR pathway through inhibition of Akt phosphorylation via phosphorylation of Smad2 and Smad3, and by down-regulating calcineurin signaling as well as the transcription factors MyoD and myogenin (Amirouche et al. 2008; McFarlane et al. 2006; Michel et al. 2007). IGF-I has been shown to block the effects of myostatin on Akt when applied to myoblasts or myotubes, suggesting that IGF-I/Akt signaling is dominant over myostatin-induced activation of Akt (Trendelenburg et al. 2009). Although the actions of myostatin are yet to be completely understood, these data indicate that myostatin negatively regulates muscle size by decreasing Akt-induced protein synthesis as well as by stimulating protein degradation via a FOXO-dependent mechanism. Whether myostatin also affects functional characteristics such as force generation is still under debate (Amthor et al. 2007; Mendias et al. 2006).
Mechanical loading has been shown to up-regulate the expression of different IGF-I isoforms up to 15-fold either alone or in combination with sustained electrical stimulation (Heinemeier et al. 2007; McKoy et al. 1999). In contrast, myostatin expression decreased two- to eightfold in response to different types of contractile activity, the effect being largest during eccentric contractions (Heinemeier et al. 2007). This suggests that mechanical loading is an important stimulus for regulating fiber size, which is mediated through increased expression of IGF-I combined with down-regulation of myostatin. New experimental PCR data in this paper show that, when normalized to total RNA, IGF-I mRNA expression levels showed no differences between high oxidative rat soleus and low oxidative EDL muscles. In contrast, mean myostatin expression levels were 6.5-fold higher in the EDL (Fig. 4a). Because the soleus contains 2.3-fold more total RNA compared to EDL (Fig. 2a), we also normalized IGF-I and myostatin mRNA expression relative to muscle mass. After normalizing in this way, IGF-I mRNA levels were 2.5-fold higher in soleus compared to EDL, whereas myostatin mRNA levels were 2.8-fold higher in the EDL (Fig. 4b). The latter result supports the data of others showing that myostatin, myostatin receptor and FOXO1 mRNA levels were higher in low oxidative muscle fibers (Allen and Unterman 2007; Atherton et al. 2004; Carlson et al. 1999). These data indicate that IGF-I expression is higher in high oxidative fibers, whereas myostatin and FOXO levels are relatively high in low oxidative fibers.
Differences in mRNA concentrations of insulin-like growth factor-I (IGF-1, all isoforms) and myostatin in rat soleus (SO) and extensor digitorum longus (EDL) muscles (n = 6) (for methods see supplementary section I). a mRNA normalized to total RNA relative to EDL. No differences between SO and EDL were found for IGF-1, but myostatin mRNA was 6.5-fold lower in SO (p < 0.001). b mRNA normalized to muscle tissue weight relative to EDL. IGF-1 expression was 2.5-fold higher in SO, whereas myostatin was 2.8-fold lower in SO (p < 0.002). Asterisks indicate significant difference compared to EDL
In addition to mechanical loading-induced expression of local factors, several other components such as albumin, IGF-binding proteins, receptors, cytokines (i.e., TNF-1α, IL-1 and IL-6), calcium and vitamins may modulate the effects of IGF-I and myostatin (Casse et al. 2003; Clemmons 1998; Coletti et al. 2005; Jaspers et al. 2008; Ling et al. 1997; Pfeifer et al. 2002). For example, insulin receptor phosphorylation, insulin receptor substrate (IRS-2) phosphorylation, as well as the downstream PI3K activity were all shown to be significantly higher in the high oxidative soleus compared to EDL (Song et al. 1999). As IGF-I also binds the insulin receptor (Shimizu et al. 1986) and IGF-I expression is higher in high oxidative fibers (Fig. 4b), these results confirm the greater IGF-I binding capacity in high oxidative fibers. These results indicate relatively high expression levels of IGF-I mRNA, its receptor and downstream signaling components in high oxidative muscle fibers, while myostatin expression is low in these fiber types. Whether these differential expression levels implicate related local effects on the producing muscle fiber remains to be determined. Very little is known about the concentrations and activity of growth factors in the vicinity of the sarcolemma. Do the growth factors diffuse and is there a gradient from one fiber to the other? With what affinity do growth factors bind to their receptors or binding proteins that enhance or attenuate their function? There are indications that growth factors bind to the glycosaminoglycans in the heparan sulfates, which may facilitate their activity at their receptor (Roghani et al. 1994). As the growth factors may also have paracrine effects, the higher IGF-I expression in high oxidative fibers may affect high oxidative and also neighboring low oxidative fibers. The magnitude of autocrine and paracrine effects warrants further research. Information is required on expression and activity of the growth factors such as IGF-I and myostatin, as well as on the expression levels of their receptors (IGF-IR and activin receptors), smad proteins and the inhibitory binding proteins (e.g., IGFBP-4, follistatin, myostatin propetide), which may enhance or attenuate the function of these growth factors.
Cytokines
Several cytokines have been reported to play a role in muscle adaptation. Interleukin (IL)-6 is released in muscle tissue in response to sustained exercise and contributes to increased AMPK and calcineurin activity and subsequent high oxidative gene expression (Banzet et al. 2005) (Fig. 3). IL-6 also has been reported to stimulate satellite cell proliferation (Austin and Burgess 1991). In contrast, tumor necrosis factor (TNF)-α, IL-1α and IL-1β promote muscle catabolism (Flores et al. 1989; Zamir et al. 1993). TNF-α stimulates the expression of the ubiquitin-ligase MAFbx via p38 and both TNF-α and IL-1 induce increased ROS production, activation of the NF-κB pathway and subsequent protein loss (Jackman and Kandarian 2004; Li et al. 2005) (Fig. 3). In addition, TNF-α increases myonuclear apoptosis in myotubes and in mouse skeletal muscle, TNF-α appears to inhibit muscle regeneration by inhibiting embryonic MyHC expression (Coletti et al. 2005; Vescovo and Dalla Libera 2006).
In human skeletal muscle, IL-6 expression is relatively high in the high oxidative fibers (Plomgaard et al. 2005). Although no fiber type-specific expression of TNF-α was found in young rats, TNF-α expression increased more with age in the low oxidative rat vastus lateralis muscle compared to the high oxidative soleus. This suggests a fiber type-dependent regulation of TNF-α with age (Phillips and Leeuwenburgh 2005). Based on the reported differences in fiber type-specific expression of cytokines, and the potential role that cytokines play in regulating protein turnover, it is tempting to speculate that cytokines play a role in regulating the fiber type-specific differences in protein turnover. This assumes that fiber type-specific differences in protein turnover exist and can be demonstrated experimentally.
Intracellular calcium
Intracellular calcium concentrations are increased by stimuli such as action potentials, stretch and/or hypoxia (Allen et al. 2005; Eltit et al. 2004; Powers et al. 2007), which release calcium from intracellular stores (sarcoplasmatic reticulum), or by growth factor signaling (e.g. IGF-I, see above). Rises in [Ca2+]i trigger several processes involved in fiber type-specific gene expression (Fig. 3). [Ca2+]i induces transcriptional activation through the calcineurin/CaMK pathways, the MAPK pathways and increased expression of transcription factors c-fos and c-jun, which are members of the immediate early gene family transiently and rapidly activated in many processes ranging from differentiation and cell growth to hypertrophy (Carrasco et al. 2003; Chin et al. 1998; Ruwhof and van der Laarse 2000). In addition, it has also been suggested that the Akt–mTOR pathway is sensitive to [Ca2+]i. Whether this effect of calcium is mediated by mechanical loading or IGF-I is unclear. Several mechanisms are known via which calcium could affect the Akt–mTOR signaling pathway, including vacuolar protein sorting mutant 34 (Vps34) and amino acid exposure (Gulati et al. 2008; MacKenzie et al. 2009). Furthermore, calcium/calcineurin-induced IGF-I expression provides another potential link to Akt–mTOR via PI3K (Alfieri et al. 2007). Protein degradation is also partly controlled by [Ca2+]i as it increases the activity of Ca2+-mediated proteases and the proteasome system (Powers et al. 2007). Although the regulation of [Ca2+]i in heart and skeletal muscle is complex, levels of resting [Ca2+]i appear to be higher in high oxidative fibers (Batkai et al. 1999). This suggests that calcium-induced protein synthesis and degradation are higher in high compared to low oxidative fibers.
Collectively, mechanical stimuli are important regulators of muscle mass, although the mechanisms connecting the mechanical events onto the intracellular signaling structure are complex and warrant further investigation. It may well be that the onset of mechanical load induces both acute and delayed signals regulating protein synthesis and degradation. For example, the acute onset of mechanical loading induces significant and immediate increases in Akt phosphorylation, which returns to baseline within 3 h after cessation of the load (Nader and Esser 2001). Such acute effects in response to mechanical loading may be mediated via integrin-FAK, Ca2+-mediated signaling, Vps34 and/or PLD. In humans IGF-I mRNA expression is up-regulated 2.5 h after a single bout of resistance exercise and persists for 2 days, suggesting that growth factors comprise a mechanism involved in the delayed onset of signaling pathways that control protein synthesis (Greig et al. 2006). Resistance exercise resulted in an immediate (1–4 h) and concurrent up-regulation of markers involved in protein synthesis (mTOR, p70S6K) and protein degradation (MuRF, but not MAFbx) in human muscles (Louis et al. 2007; Mascher et al. 2008). However, the acute response was followed by a delayed (>8 h) decrease in markers for degradation, whereas the markers for protein synthesis were stable (Louis et al. 2007; Mascher et al. 2008). Also, after a longer period of resistance exercise, it was shown that down-regulation of proteolytic markers persisted, whereas synthetic markers were similar compared to pre-exercise (Mascher et al. 2008; Zanchi et al. 2009). In contrast, endurance exercise in humans increased the proteolytic factors MAFbx, MuRF and FOXO directly (1–4 h after exercise), while long-term effects were unclear (Louis et al. 2007). It appears that different exercise protocols result in the selective activation of specific intracellular signaling pathways, which are most likely responsible for a concurrent training effect (see above). It remains to be determined in what way the acute or delayed onset of signaling pathways in response to mechanical load affect the balance between protein synthesis and degradation, and whether such responses are fiber-type specific.
Cellular energy status
The availability of cellular energy (ΔG ATP) is predominantly maintained by mitochondria. It is generally assumed that the ratio between AMP and ATP (AMP:ATP) reflects the energy status of a muscle cell and acts as a major stimulus for AMPK (Hardie and Sakamoto 2006). An increase in AMP:ATP (e.g., caused by contractile activity or hypoxia) leads to robust activation of AMPK, which attenuates the translational signaling, while at the same time gene expression associated with high oxidative metabolism and mitochondrial biogenesis are enhanced and NF-κB–proteasome-mediated degradation is increased (Fig. 3).
In general, mitochondrial proteins turn over with a half-life between 4 and 8 days with half-life being shortest in the high oxidative fibers, although turnover of cytochrome c was higher in the low oxidative fibers (Booth and Holloszy 1977; Hickson and Rosenkoetter 1981; Terjung 1979; Wicks and Hood 1991). Based on these data, it may be speculated that a continuous stimulus for AMPK activation is required to maintain mitochondrial content at an elevated level; otherwise, a loss of oxidative capacity will result. Low cellular energy levels, which are generally induced in response to sustained contractile activity or hypoxia, will activate AMPK. As AMPK simultaneously reduces translational processes while increasing high oxidative gene expression and the rate of protein degradation, a low energy status would be associated with a high rate of protein turnover that limits the potential to increase fiber size.
Cellular oxygen levels
Assuming that the half saturation pressure of myoglobin is 2.75 mmHg (Rossi-Fanelli and Antonini 1958) and 90% of myoglobin is oxygenated at rest (Richardson et al. 2006), it can be estimated that the average muscle fiber PO2 in humans equals 25 mmHg at rest. As mentioned above, an interstitial oxygen tension of about 14 mmHg is required to prevent anoxic cores (and impaired mitochondrial respiration) at the maximum rate of oxygen consumption. Therefore, cellular oxygen levels play a critical role in regulating muscle protein turnover (see oxidative stress-mediated proteolysis, Suppl. Fig. 2D, Fig. 3). Hypoxia increases ROS production and affects mitochondrial oxygen uptake and permeability, which reduces cellular ATP production and activates AMPK. In myotubes, in response to hypoxia, the rate of protein degradation was elevated and the rate of protein synthesis reduced, explaining the atrophic effects of hypoxia (Caron et al. 2009).
The effects of hypoxia on fiber size are at least in part mediated via ROS production, which is likely to exceed antioxidant production during prolonged periods of hypoxia, resulting in an elevated rate of protein degradation (Powers et al. 1999). High oxidative fibers generally consume more oxygen compared to low oxidative fibers and therefore it is assumed that their rates of ROS and antioxidant production are relatively high compared to low oxidative fibers (Malinska et al. 2009; Powers et al. 1999). Because ROS are scavenged by antioxidants, this does not necessarily imply that the ROS concentrations differ between fiber types, although there are indications that specific types of radicals may vary between high and low oxidative fibers (Anderson and Neufer 2006). The specific function of free radicals may also differ between fiber types. An example of this is nitric oxide (NO), a relatively well-studied free radical in muscle. NO is produced by various NO synthase isoforms (NOS), such as neuronal NOS (nNOS) and endothelial NOS (eNOS), which are permanently expressed in skeletal muscle. The data on NOS are conflicting. For example in humans, nNOS protein levels are similar between fiber types, but increase in all fiber types in response to exercise (McConell et al. 2007). Also, higher as well as lower concentrations of nNOS have been reported in high oxidative fibers, depending on the species (e.g., (Frandsen et al. 1996; Kobzik et al. 1994). In addition, the production of NO not only depends on expression levels of NOS, but also on the activity of different isoforms, which can be modulated by calcium and calmodulin (Nathan and Xie 1994). In cachectic mice, muscle NO production and subsequent activation of antioxidant genes were higher in high oxidative muscle fibers, thereby protecting these fibers against muscle wasting (Yu et al. 2008). This suggests that NO leads to increased ROS scavenging and plays a protective role by inhibiting expression of E3 ligases and protein degradation. In addition, NO plays a role in mitochondrial biogenesis as well as in preventing exercise-related membrane damage and breakdown of structural muscle protein, while in myoblasts NO inhibits calpain-mediated proteolysis of cytoskeletal proteins (Koh and Tidball 1999; Nguyen and Tidball 2003; Nisoli et al. 2003). However, NO can also react with superoxide anion to produce the highly toxic peroxynitrite, which is associated with enhanced protein degradation and apoptosis (Ghafourifar and Cadenas 2005). These data show that the role of free radicals, such as NO, in regulating protein turnover remains ambiguous, despite fiber type-specific differences in concentration.
Cellular oxygen supply can be improved by increasing capillarization, hematocrit or myoglobin concentration (Des Tombe et al. 2002; van Beek-Harmsen et al. 2004). The signal transduction mechanisms that regulate oxygen supply have been shown to involve the hypoxia-inducible factor-1 (HIF-1α), which mediates expression of erythropoietin and angiogenic growth factors such as vascular endothelial growth factor (VEGF) (Hirota and Semenza 2006). VEGF up-regulates myoglobin in vivo and in vitro and as a result HIF-1α also improves tissue oxygenation in the absence of improved vascularization (Hirota and Semenza 2006; van Weel et al. 2004). In addition, hypoxia-induced calcium transients have been shown to induce myoglobin expression in mice (Kanatous et al. 2009). Besides facilitation of oxygen transport, oxyhemoglobin and myoglobin act as scavengers for NO (Brandish et al. 1998; Flogel et al. 2001). There is some debate on whether oxyhemoglobin and myoglobin can reduce NO levels partially or completely in vivo (Hazarika et al. 2008; Kreutzer and Jue 2004). However, since NO increases ROS scavenging and subsequent proteasome activity (Yu et al. 2008), it may be hypothesized that a higher oxyhemoglobin/myoglobin concentration is linked to an increased ROS-mediated protein degradation. If so, increasing myoglobin may increase the oxidative capacity, but it also stimulates protein degradation, which limits the fiber size at a constant rate of protein synthesis.
Alternative strategies for improving oxygen supply may include alteration of mitochondrial distribution, for example by relocating the mitochondria to the sarcolemma (Hardy et al. 2009). However, a predominantly subsarcolemmal distribution is associated with large intracellular diffusion distances for metabolites, which would have functional consequences (Kinsey et al. 2007) and may also be associated with pathological features such as muscle weakness and hypotonia (Reid and MacGowan 1998).
In conclusion, hypoxia results in elevated ROS levels and reduced cellular ATP levels, concomitant with an increased degradation and decreased synthesis of muscle protein. Although both ROS and antioxidant production differ between fiber types, the cumulative and relative effects of various ROS concentrations on the regulation of protein turnover in different fiber types are not clear yet. However, it appears that ROS do play a role in hypoxia-induced reduction in fiber size. In addition, it is likely that hypoxia affects fiber size via other fiber type-specific mechanisms that are induced by the low cellular energy status during hypoxia.
Conclusions
Ever since the classical training experiments of Hickson (1980), we know that human skeletal muscles have limited capacity to improve strength and fatigue resistance at the same time. This observation is valid for striated muscle tissue, in general, as an inverse relationship exists between muscle fiber size and its oxidative capacity (Fig. 1). This relationship suggests that striated muscle cells are evolved in such a way that anoxic cores are prevented. The central question addressed in this review is: ‘why do the high oxidative fibers remain small compared to the low oxidative fibers’?
At first sight it appears paradoxical that high oxidative fibers are relatively small, since these fibers have a larger capacity for protein synthesis compared to low oxidative fibers. All major components of the transcription machinery (i.e., satellite cells, myonuclei, mitochondria and mRNA) as well as total ribosomal RNA content are present in higher quantities in high oxidative fibers. This paradox may not be a quantitative one, as there is a discrepancy in mass of large myofibrillar proteins and much smaller oxidative proteins. However, another paradoxical outcome of this review is that IGF-I expression, a potent stimulator of myofibrillar protein synthesis and hypertrophy (Bodine et al. 2001b; Stitt et al. 2004), is also higher in high oxidative muscles, whereas myostatin, an endogenous inhibitor of muscle mass (Lee and McPherron 2001; Zimmers et al. 2002), shows higher expression in low oxidative muscles (Fig. 4).
Several critical stimuli, such as contractile activity, mechanical loading, cellular energy levels and hypoxia, control the activity status of signaling pathways involved in regulating fiber size in high and low oxidative fibers (Fig. 3). The rate of synthesis is largely regulated by the Akt–mTOR pathway, which induces hypertrophy in response to short contractions, mechanical loading and regulation and presence or absence of growth factors such as IGF-I and myostatin, respectively. In contrast, hypoxia and sustained contractile activity, associated with low cellular energy levels, activate Ca2+-calcineurin and AMPK. Both calcineurin and AMPK stimulate oxidative metabolism and slow-type gene expression through PGC-1α, while AMPK simultaneously attenuates protein synthesis by inhibiting mTOR, via activation of TSC-2. Akt activation has been shown to restore cellular energy levels via an increase in glycolysis and oxidative phosphorylation, thereby inactivating AMPK (Gottlob et al. 2001; Hahn-Windgassen et al. 2005). Therefore, the competitive interaction between Akt–mTOR and AMPK acts as a ‘switch’ that controls the synthesis of myofibrillar protein and metabolic protein (Fig. 3) (Atheron et al. 2005; Baar 2006). It has been shown that activation of TSC-2 by AMPK is dominant over Akt-induced inactivation of TSC-2 and leads to inactivation of mTOR (Inoki et al. 2003). This implies that the cellular energy status may be crucial in mediating either a low oxidative phenotype and a large size or a high oxidative phenotype and a small size. However, currently we do not know whether the ‘Akt–AMPK switch’ is sufficient to explain why high oxidative fibers show such limited hypertrophy, largely because mTOR can be activated independently of Akt and mTOR also appears to be required for the regulation of oxidative metabolism (Bentzinger et al. 2008; Cunningham et al. 2007; Fang et al. 2001; Hornberger et al. 2006). In addition, recent experiments on humans showed that, for example, training history and nutritional status (amino acid and carbohydrate ingestion) influence the molecular response to different types of contractile activity (resistance vs. endurance) (Coffey et al. 2006; Deldicque et al. 2008). To link various stimuli to the regulation of fiber size, one needs to know the relative contribution of each pathway involved in myofibrillar protein synthesis and mitochondrial biogenesis in different fiber types. The canonical Akt/mTOR–AMPK switch is merely a mechanism controlling the synthesis of myofibrillar and oxidative protein and may therefore only partly explain the relatively small size of the high oxidative fibers. As the balance between synthesis and degradation determines whether protein is gained or lost, the rate of protein degradation also largely contributes to regulating the fiber size.
Despite the still limited information regarding the regulation of protein degradation in different fiber types, experimental data in this paper show that several components of the degradation machinery (i.e., expression levels of ubiquitin-ligases MAFbx and MuRF, Fig. 2) are about twofold higher in high oxidative fibers. These data support the hypothesis that the high rate of protein degradation in high oxidative fibers is balanced by a high rate of synthesis, thereby creating a steady state protein turnover. However, if the rate of degradation becomes higher (e.g., due to low energy status or high ROS production), such as that in the more oxidative fibers, this would require an increasingly high rate of synthesis to maintain the steady state. Therefore, the relatively high rate of degradation in high oxidative fibers may be an important factor limiting the size of these fibers.
The rate of protein degradation is at least in part regulated by growth factor signaling. IGF-I and myostatin concentrations are differentially regulated in high and low oxidative fibers (Fig. 4), and in response to contractile activity their actions both have a hypertrophic effect. IGF-I stimulates, whereas myostatin inhibits, Akt (Fig. 3); therefore, it can be expected that phosphorylated Akt levels are higher in high oxidative fibers. The single study reporting on total and phosphorylated Akt concentrations in high and low oxidative muscles confirmed that both total and phosphorylated Akt were higher in the high oxidative soleus compared to the low oxidative EDL (Sakamoto et al. 2002). However, Akt phosphorylates FOXO, resulting in translocation of FOXO out of the nucleus and a decreased transcription of FOXO target genes, MAFbx and MuRF. Although there are no quantitative data available on total and phosphorylated FOXO protein concentrations, FOXO mRNA was shown to be higher in low oxidative muscles, when normalized to β-actin (Allen and Unterman 2007). Our data show that high oxidative soleus muscle contains two times more total RNA per microgram muscle tissue compared to the low oxidative EDL (Fig. 2a). We have shown that when normalized per muscle tissue weight, EDL shows twofold higher myostatin expression (Fig. 4b), indicating that myostatin mRNA is higher in low oxidative fibers. Myostatin has been shown to induce expression and nuclear accumulation of FOXO (McFarlane et al. 2006). As FOXO binds and activates the myostatin promoter, it functions in a feed-forward loop with myostatin (Allen and Unterman 2007). These data suggest that myostatin and nuclear FOXO concentrations are higher in the low oxidative fibers. As nuclear localization of FOXO enhances the expression of MAFbx and MuRF (Sandri et al. 2004), higher expression levels of these E3 ligases are expected in low oxidative fibers. We found that when normalized to total RNA, MAFbx and MuRF expression were similar in soleus and EDL muscles (Fig. 2b). However, as the soleus contains about twofold more total RNA compared to EDL, normalizing relative to muscle tissue weight showed that MAFbx and MuRF expression were both about twofold higher in the soleus (Fig. 2c). This indicates that the higher expression of E3 ligases in high oxidative fibers may be due to other signaling routes besides FOXO. The most likely candidates for such alternative routes are the AMPK–NF-κB pathway and the p38 MAPK–NF-κB pathway (Fig. 3). The relatively high levels of NF-κB expression in high oxidative muscles (Atherton et al. 2004; Phillips and Leeuwenburgh 2005) combined with higher expression levels of MAFbx and MuRF in high oxidative muscles (Fig. 2) support this hypothesis.
Considerable progress has been made in elucidating the signaling mechanisms and their interactions that control protein synthesis and degradation in skeletal muscle. In Fig. 5, these interactions are integrated into a model for the regulation of fiber size and oxidative metabolism. In general, sustained low-intensity activity results in a reduced cellular energy status (AMP:ATP), hypoxia, elevated [Ca2+]i and increased IGF-I expression, whereas short bursts of activity and at high-intensity mechanical loads induce transient changes in [Ca2+]i and increased expression of growth factors. These types of contractile activity will induce fiber type-specific signaling pathways that enhance the rates of protein synthesis and degradation, or both. The relative concentrations of structural muscle proteins or metabolic proteins largely depend on the interaction between pathways at the ‘Akt/mTOR–AMPK switch’ (Atheron et al. 2005; Baar 2006). In addition, the regulation of cellular oxygen and energy levels determine the impact of protein degradation on the rate of turnover. Conditions of hypoxia and low energy status induce ROS- and AMPK-mediated NF-κB activity. The subsequent increase in the rate of myofibrillar degradation limits fiber size, which facilitates the restoration of cellular energy status and oxygen tension and prevents hypoxia. In contrast, restoration of cellar energy levels attenuates AMPK activation and subsequently the rate of protein degradation and the capacity to sustain mitochondrial biogenesis and oxidative metabolism via AMPK–PGC1α. However, the oxidative metabolism is also increased by activation of mTOR, which has been related to the significant amounts of energy required for anabolic actions such as protein synthesis (Cunningham et al. 2007). Therefore, it appears that hypoxia and low cellular energy levels reduce the synthesis, while simultaneously increasing the degradation of muscle protein. In contrast, normoxic conditions and high cellular energy levels would facilitate hypertrophy by inducing protein synthesis and by limiting the rate of degradation.
Schematic diagram representing the regulation of muscle fiber size and oxidative metabolism. The type of contractile activity, ranging from sustained low-level activity to short high-level activity, combined with the cellular energy (AMP:ATP) and oxygen (ROS concentration) status determine the rate of synthesis and degradation of both contractile and metabolic protein. The balance between synthesis and degradation reflects the rate of protein turnover and determines whether contractile or metabolic protein is gained or lost, thereby regulating the fiber size. Solid lines are dominant processes in high oxidative fibers, whereas dashed lines are dominant in low oxidative fibers. The plus and minus signs reflect the effects of the different processes (e.g., synthesis, degradation, hypoxia) on fiber size and mitochondrial density (for details see text)
Currently, we are beginning to understand how different types of contractile activity and mechanical loading interact and affect the activation of pathways involved in protein synthesis and degradation. However, the relative contribution of the pathways in high and low oxidative fibers, the effect of nutritional status (e.g., amino acids and carbohydrates) and also the time frames of the responses that convert mechanical events at the sarcolemma onto the intracellular signaling structure are still poorly understood. In addition, it should be realized that an increase in muscle mRNA in response to activity or mechanical load does not necessarily induce` a higher protein production. The relationship between mRNA variation and protein synthesis should be determined systematically in response to various types of activity and mechanical loading: because it appears that timing of both the synthetic and proteolytic response are differently regulated, depending on the type of exercise and duration (Jensen et al. 2009). With this in mind, several potential mechanisms may account for the differences in up-regulation of gene and protein expression involved in the hypertrophic response that need to be confirmed. Growth factors show a delayed induction in response to changes in mechanical loading, whereas growth factor-independent mechanisms, such as signaling via integrin-FAK, Ca2+, amino acids, Vps34 and/or PLD, may be responsible for the early induction of protein synthesis. In our view, the potential mechanisms that can have a major effect on the fiber type-specific regulation of proteolytic factors in response to various types of exercise comprise the AMPK–NF-κB pathway, the p38 MAPK–NF-κB pathway and the Ca2+-mediated proteasome pathway. Understanding the fiber type and timing-related differences in protein synthesis and degradation requires an integrative approach, combining in vitro in situ as well as in vivo studies. Quantitative fiber type differentiating approaches obtain insight into interactions between pathways and the integrated effects of different loading programs and stimuli. For example, ex vivo culture systems of mature muscle fibers (Jaspers 2004; Jaspers et al. 2008) are well defined and can be used for controlled manipulation of growth factors, mechanical loading and oxygen tension, either independently or in combination. Such approaches may uncover interactions between signal transduction routes, which are to be verified in vivo. Furthermore, to assess fiber type-specific absolute total and phosphorylated concentrations of the major signaling molecules involved in muscle protein turnover, quantitative immunohistological methods or in situ hybridizations (van Beek-Harmsen et al. 2004; van Beek-Harmsen and van der Laarse 2005) are required. These methods allow the identification of the activity status of pathways inducing a high or low oxidative phenotype and clarify the impact of the pathways and stimuli on the muscle fiber size.
Perspectives and significance
It is a challenge to optimize training and rehabilitation programs with respect to the functional demands. To design the appropriate training and rehabilitation strategies, one needs to understand the interactions between signaling pathways for myofibrillar protein synthesis, mitochondrial biosynthesis and degradation. Understanding in what way oxygen supply (in terms of increased capillarization, hematocrit and expression of myoglobin) can be improved without compromising the capacity for force production is another challenge. For instance, when both high peak power and high steady state power are required, such as in the final sprint of a cycling stage or a marathon, one would need to design a training strategy inducing large fibers with high capacity for oxygen transport to sustain a high steady state power, as well as large glycogen stores to maintain a high anaerobic power during the sprint. To maintain a certain size, cellular oxygen tension and ROS must be kept within a critical range of beneficial concentrations and cellular energy levels may not be reduced. A small disturbance in oxygen or energy levels, either up or downward, by contractile activity or hypertrophy, will result in adaptation of the fiber size and the oxidative metabolism. Whether a muscle fiber is actually capable of increasing its size and becoming stronger while maintaining endurance capacity depends primarily on: (1) the application of appropriate stimuli (i.e., sustained contractile activity combined with short, powerful mechanical loading) and availability of the machinery and essential substrates (e.g., amino acids) to maintain the rate of muscle protein synthesis; (2) the capacity to increase oxygen transport (e.g., by improving heart and lung function or angiogenesis, hematocrit and myoglobin); and (3) prevention of tissue hypoxia and chronically reduced cellular energy status. For patients suffering from chronic diseases, such as heart failure and COPD, or for sportsmen and women exploring their physical limits, it implies that one must know the status of the above-mentioned determinants to develop an appropriate strategy to improve their performance. Furthermore, quantitative information and sophisticated mathematical models are required to predict the outcome of manipulations of interacting signal transduction routes.
Abbreviations
- α M :
-
Solubility of oxygen in muscle
- ΔG ATP :
-
Gibbs energy of ATP hydrolysis determined by the cytosolic phosphorylation ratio ([ATP]/[ADP][Pi])
- [Ca2+]i :
-
Intracellular calcium concentration
- 4E-BP1:
-
EIF-2 binding protein1
- ADP:
-
Adenosine diphosphate
- AMP:
-
Adenosine monophosphate
- AMPK:
-
AMP-activated protein kinase
- ATP:
-
Adenosine triphosphate
- bFGF:
-
Basic fibroblast growth factor
- CaMK:
-
Calcium/calmodulin protein kinase
- CSA:
-
Cross-sectional area
- DO2:
-
The diffusion coefficient for oxygen in sarcoplasm
- EDL:
-
Extensor digitorum longus muscle
- eEF:
-
Eukaryotic elongation factor
- eIF:
-
Eukaryotic initiation factor
- eNOS:
-
Endothelial nitric oxide synthase
- ERK:
-
Extracellular regulated kinase
- FAC:
-
Integrin-focal adhesion complex
- FAK:
-
Focal adhesion kinase
- FOXO:
-
Forkhead box transcription factors O
- GAPDH:
-
Glyceralde-3 phosphate dehydrogenase.
- GSK-3β:
-
Glycogen synthase kinase-3β
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor-1α
- IGF-I:
-
Insulin-like growth factor-I
- IGFBP:
-
Insulin-like growth factor binding protein
- IGF-IR:
-
Insulin-like growth factor receptor
- IL:
-
Interleukin
- IRS-2:
-
Insulin receptor substrate-2
- JNK:
-
c-Jun N-terminal kinase
- MAFbx:
-
Muscle atrophy F-box
- MAPK:
-
Mitogen-activated protein kinase
- MEF-2:
-
Myocyte enhancer factor-2
- MGF:
-
Mechano-growth factor
- miRNA:
-
Micro RNA
- mRNA:
-
Messenger RNA
- mtDNA:
-
Mitochondrial DNA
- mtmRNA:
-
Mitochondrial mRNA
- mTOR:
-
Mammalian target of rapamycin
- mtrRNA:
-
Mitochondrial rRNA
- MuRF:
-
Muscle ring finger
- MyHC:
-
Myosin heavy chain
- nDNA:
-
Nuclear DNA
- NF-κB:
-
Nuclear factor kappa-B
- NFAT:
-
Nuclear factor of activated T cells
- nNOS:
-
Neuronal nitric oxide synthase
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxide synthase
- PGC-1α:
-
Peroxisome proliferator-activated receptor γ co-activator 1α
- p38:
-
38 kDa stress-activated protein kinase
- p70S6K:
-
70-kDa ribosomal protein S6 kinase
- PA:
-
Phosphatidic acid
- PCR:
-
Polymerase chain reaction
- Pi :
-
Intracellular phosphate
- PI3K:
-
Phosphatidylinositol 3-kinase
- PLD:
-
Phospholipase D
- PO2:
-
Interstitial oxygen tension
- RNS:
-
Reactive nitrogen species
- ROS:
-
Reactive oxygen species
- rRNA:
-
Ribosomal RNA
- SAC:
-
Stretch-activated channel
- SDH:
-
Succinate dehydrogenase
- SRF:
-
Serum response factor
- TGF-β:
-
Transforming growth factor-β
- TNF-α:
-
Tumor necrosis factor-α
- tRNA:
-
Transfer RNA
- TSC-2:
-
Tuberous sclerosis complex-2
- VEGF:
-
Vascular endothelial growth factor
- VO2max:
-
Maximum rate of oxygen consumption
- Vps34:
-
Vacuolar protein sorting mutant 34
References
Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z (2005) Exercise stimulates Pgc-1 alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem 280:19587–19593
Alfieri CM, Evans-Anderson HJ, Yutzey KE (2007) Developmental regulation of the mouse IGF-I exon 1 promoter region by calcineurin activation of NFAT in skeletal muscle. Am J Physiol Cell Physiol 292:C1887–C1894
Allen RE, Boxhorn LK (1989) Regulation of skeletal-muscle satellite cell-proliferation and differentiation by transforming growth factor-beta, insulin-like growth factor-I, and fibroblast growth-factor. J Cell Physiol 138:311–315
Allen DL, Unterman TG (2007) Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am J Physiol Cell Physiol 292:C188–C199
Allen DL, Monke SR, Talmadge RJ, Roy RR, Edgerton VR (1995) Plasticity of myonuclear number in hypertrophied and atrophied mammalian skeletal-muscle fibers. J Appl Physiol 78:1969–1976
Allen DG, Whitehead NP, Yeung EW (2005) Mechanisms of stretch-induced muscle damage in normal and dystrophic muscle: role of ionic changes. J Physiol 567:723–735
Alway SE, Macdougall JD, Sale DG, Sutton JR, McComas AJ (1988) Functional and structural adaptations in skeletal-muscle of trained athletes. J Appl Physiol 64:1114–1120
Amirouche A, Durieux AC, Banzet S, Koulmann N, Bonnefoy R, Mouret C, Bigard X, Peinnequin A, Freyssenet D (2008) Down-regulation of Akt/mTOR signaling pathway in response to myostatin overexpression in skeletal muscle. Endocrinol (E-pub ahead of print)
Amthor H, Macharia R, Navarrete R, Schuelke M, Brown SC, Otto A, Voit T, Muntoni F, Vrbova G, Partridge T, Zammit P, Bunger L, Patel K (2007) Lack of myostatin results in excessive muscle growth but impaired force generation. PNAS 104:1835–1840
Andersen LL, Suetta C, Andersen JL, Kjaer M, Sjogaard G (2008) Increased proportion of megafibers in chronically painful muscles. Pain 139:588–593
Anderson EJ, Neufer PD (2006) Type II skeletal myofibers possess unique properties that potentiate mitochondrial H2O2 generation. Am J Physiol Cell Physiol 290:C844–C851
Arany Z, Lebrasseur N, Morris C, Smith E, Yang WL, Ma YH, Chin S, Spiegelman BM (2007) The transcriptional coactivator PGC-1 beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab 5:35–46
Armstrong RB, Phelps RO (1984) Muscle-fiber type composition of the rat hindlimb. Am J Anat 171:259–272
Atheron PJ, Babraj JA, Smith K, Singh J, Rennie MJ, Wackerhage H (2005) Selective activation of AMPK–PGC-1 alpha or PKB–TSC2–mTOR signaling can explain specific adaptive responses to endurance or resistance training-like electrical muscle stimulation. FASEB J 19:786–788
Atherton PJ, Higginson JM, Singh J, Wackerhage H (2004) Concentrations of signal transduction proteins mediating exercise and insulin responses in rat extensor digitorum longus and soleus muscles. Mol Cell Biochem 261:111–116
Austin L, Burgess AW (1991) Stimulation of myoblast proliferation in culture by leukemia inhibitory factor and other cytokines. J Neurol Sci 101:193–197
Baar K (2006) Training for endurance and strength: lessons from cell signaling. Med Sci Sports Exerc 38:1939–1944
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol Cell Physiol 276:C120–C127
Banzet S, Koulmann N, Simler N, Birot O, Sanchez H, Chapot R, Peinnequin A, Bigard X (2005) Fibre-type specificity of interleukin-6 gene transcription during muscle contraction in rat: association with calcineurin activity. J Physiol 566:839–847
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bas A, Forsberg G, Hammarstrom S, Hammarstrom ML (2004) Utility of the housekeeping genes 18S rRNA, beta-actin and glyceraldehyde-3-phosphate-dehydrogenase for normalization in real-time quantitative reverse transcriptase-polymerase chain reaction analysis of gene expression in human T lymphocytes. Scand J Immunol 59:566–573
Batkai S, Racz IB, Ivanics T, Toth A, Hamar J, Slaaf DW, Reneman RS, Ligeti L (1999) An in vivo model for studying the dynamics of intracellular free calcium changes in slow- and fast-twitch muscle fibres. Pflugers Arch-Eur J Physiol 438:665–670
Bechet D, Tassa A, Taillandier D, Cornbaret L, Attaix D (2005) Lysosomal proteolysis in skeletal muscle. Int J Biochem Cell Biol 37:2098–2114
Bekedam MA, van Beek-Harmsen BJ, Boonstra A, van Mechelen W, Visser FC, van der Laarse WJ (2003) Maximum rate of oxygen consumption related to succinate dehydrogenase activity in skeletal muscle fibres of chronic heart failure patients and controls. Clin Physiol Funct Imag 23:337–343
Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia JY, Casanova E, Costa CF, Brink M, Zorzato F, Hall MN, Ruegg MA (2008) Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab 8:411–424
Bigard AX, Brunet A, Guezennec CY, Monod H (1991) Effects of chronic hypoxia and endurance training on muscle capillarity in rats. Pflugers Arch-Eur J Physiol 419:225–229
Bigard X, Sanchez H, Zoll J, Mateo P, Rousseau V, Veksler V, Ventura-Clapier R (2000) Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J Biol Chem 275:19653–19660
Blomstrand E, Radegran G, Saltin B (1997) Maximum rate of oxygen uptake by human skeletal muscle in relation to maximal activities of enzymes in the Krebs cycle. J Physiol 501:455–460
Bodine SC, Latres E, Baumhueter S, Lai VKM, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na EQ, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ (2001a) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294:1704–1708
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001b) Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3:1014–1019
Bolster DR, Kimball SR, Jefferson LS (2003) Translational control mechanisms modulate skeletal muscle gene expression during hypertrophy. Exerc Sport Sci Rev 31:111–116
Booth FW, Holloszy JO (1977) Cytochrome-C turnover in rat skeletal-muscles. J Biol Chem 252:416–419
Boveris A, Chance B (1973) Mitochondrial generation of hydrogen-peroxide—general properties and effect of hyperbaric-oxygen. Biochem J 134:707–716
Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, Pakay JL, Parker N (2004) Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 37:755–767
Brandish PE, Buechler W, Marletta MA (1998) Regeneration of the ferrous heme of soluble guanylate cyclase from the nitric oxide complex: acceleration by thiols and oxyhemoglobin. Biochem 37:16898–16907
Burleigh IG (1977) Observations on number of nuclei within fibers of some red and white muscles. J Cell Sci 23:269–284
Burridge K, Turner CE, Romer LH (1992) Tyrosine phosphorylation of Paxillin and Pp125(Fak) accompanies cell-adhesion to extracellular-matrix—a role in cytoskeletal assembly. J Cell Biol 119:893–903
Butterfield TA, Best TM (2009) Stretch-activated ion channel blockade attenuates adaptations to eccentric exercise. Med Sci Sports Exerc 41:351–356
Cai DS, Frantz JD, Tawa NE, Melendez PA, Oh BC, Lidov HGW, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE (2004) IKK beta/NF-kappa B activation causes severe muscle wasting in mice. Cell 119:285–298
Calbet JAL, Radegran G, Boushel R, Saltin B (2009) On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: role of muscle mass. J Physiol 587:477–490
Carlson CJ, Booth FW, Gordon SE (1999) Skeletal muscle myostatin mRNA expression is fiber-type specific and increases dining hindlimb unloading. Am J Physiol-Reg Integr Comp Physiol 277:R601–R606
Caron MA, Theriault ME, Pare ME, Maltais F, Debigare R (2009) Hypoxia alters contractile protein homeostasis in L6 myotubes. FEBS Lett 583:1528–1534
Carrasco MA, Riveros N, Rios J, Muller M, Torres F, Pineda J, Lantadilla S, Jaimovich E (2003) Depolarization-induced slow calcium transients activate early genes in skeletal muscle cells. Am J Physiol Cell Physiol 284:C1438–C1447
Carson JA, Wei L (2000) Integrin signaling’s potential for mediating gene expression in hypertrophying skeletal muscle. J Appl Physiol 88:337–343
Carson JA, Schwartz RJ, Booth FW (1996) SRF and TEF-1 control of chicken skeletal alpha-actin gene during slow-muscle hypertrophy. Am J Physiol Cell Physiol 270:C1624–C1633
Casse AH, Desplanches D, Mayet-Sornay MH, Raccurt M, Jegou S, Morel G (2003) Growth hormone receptor expression in atrophying muscle fibers of rats. Endocrinol 144:3692–3697
Challoner DR (1968) Respiration in myocardium. Nature 217:78–79
Chan AYM, Dyck JRB (2005) Activation of AMP-activated protein kinase (AMPK) inhibits protein synthesis: a potential strategy to prevent the development of cardiac hypertrophy. Can J Physiol Pharmacol 83:24–28
Charvet C, Houbron C, Parlakian A, Giordani J, Lahoute C, Bertrand A, Sotiropoulos A, Renou L, Schmitt A, Melki J, Li ZL, Daegelen D, Tuil D (2006) New role for serum response factor in postnatal skeletal muscle growth and regeneration via the interleukin 4 and insulin-like growth factor 1 pathways. Mol Cell Biol 26:6664–6674
Chen JF, Mandel EM, Thomson JM, Wu QL, Callis TE, Hammond SM, Conlon FL, Wang DZ (2006) The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet 38:228–233
Chin ER (2005) Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol 99:414–423
Chin ER, Olson EN, Richardson JA, Yano Q, Humphries C, Shelton JM, Wu H, Zhu WG, Bassel-Duby R, Williams RS (1998) A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Gen Dev 12:2499–2509
Clemmons DR (1998) Role of insulin-like growth factor binding proteins in controlling IGF actions. Mol Cell Endocrinol 140:19–24
Clerk A, Sugden PH (1997) Cell stress-induced phosphorylation of ATF2 and c-Jun transcription factors in rat ventricular myocytes. Biochem J 325:801–810
Coffey VG, Shield A, Canny BJ, Carey KA, Smith D, Hawley JA (2006) Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol-Endocrinol Metab 290:E849–E855
Coletti D, Moresi V, Adamo S, Molinaro M, Sassoon D (2005) Tumor necrosis factor-alpha gene transfer induces cachexia and inhibits muscle regeneration. Genesis 43:120–128
Cross DAE, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA (1995) Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-B. Nature 378:785–789
Csukly KJ, Martineau LC, Gardiner PF (2002) Inter- and intra-muscle comparisons of MAPK mechanosensitivity: evidence for the absence of fibre-type dependency. Pflugers Arch Eur J Physiol 444:732–737
Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007) mTOR controls mitochondrial oxidative function through a YY1-PGC-1 alpha transcriptional complex. Nature 450:736–740
Dalla Libera L, Ravara B, Volterrani M, Gobbo V, Della Barbera M, Angelini A, Danieli Betto D, Germinario E, Vescovo G (2004) Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am J Physiol Cell Physiol 286:C138–C144
Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH (2007) In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 14:796–806
Davis FJ, Gupta M, Camoretti-Mercado B, Schwartz RJ, Gupta MP (2003) Calcium/calmodulin-dependent protein kinase activates serum response factor transcription activity by its dissociation from histone deacetylase, HDAC4—implications in cardiac muscle gene regulation during hypertrophy. J Biol Chem 278:20047–20058
Delbono O, Renganathan M, Messi ML (1997) Regulation of mouse skeletal muscle L-type Ca2+ channel by activation of the insulin-like growth factor-1 receptor. J Neurosci 17:6918–6928
Deldicque L, Theisen D, Francaux M (2005) Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur J Appl Physiol 94:1–10
Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M (2008) Decrease in Akt/PKB signalling in human skeletal muscle by resistance exercise. Eur J Appl Physiol 104:57–65
Des Tombe AL, Van Beek-Harmsen BJ, Lee-De Groot MBE, Van Der Laarse WJ (2002) Calibrated histochemistry applied to oxygen supply and demand in hypertrophied rat myocardium. Micr Res Tech 58:412–420
Desplanches D, Hoppeler H, Tuscher L, Mayet MH, Spielvogel H, Ferretti G, Kayser B, Leuenberger M, Grunenfelder A, Favier R (1996) Muscle tissue adaptations of high-altitude natives to training in chronic hypoxia or acute normoxia. J Appl Physiol 81:1946–1951
Deveci D, Marshall JM, Egginton S (2001) Relationship between capillary angiogenesis, fiber type, and fiber size in chronic systemic hypoxia. Am J Physiol Heart Circ Physiol 281:H241–H252
Dibb KM, Graham HK, Venetucci LA, Eisner DA, Trafford AW (2007) Analysis of cellular calcium fluxes in cardiac muscle to understand calcium homeostasis in the heart. Cell Calcium 42:503–512
Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386:855–858
Doumit ME, Cook DR, Merkel RA (1993) Fibroblast growth-factor, epidermal growth-factor, insulin-like growth-factors, and platelet-derived growth factor-Bb stimulate proliferation of clonally derived porcine myogenic satellite cells. J Cell Physiol 157:326–332
Du J, Wang XN, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE (2004) Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Investig 113:115–123
Dufour E, Ouali A, Obled A, Deval C, Valin C (1989) Lysosomal Proteinase-Sensitive Regions in Fast and Slow Skeletal-Muscle Myosins. Biochimie 71:625–632
Dunn SE, Burns JL, Michel RN (1999) Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem 274:21908–21912
Duranteau J, Chandel NS, Kulisz A, Shao ZH, Schumacker PT (1998) Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem 273:11619–11624
Eltit JM, Hidalgo J, Liberona JL, Jaimovich E (2004) Slow calcium signals after tetanic electrical stimulation in skeletal myotubes. Biophys J 86:3042–3051
Fang YM, Vilella-Bach M, Bachmann R, Flanigan A, Chen J (2001) Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science 294:1942–1945
Flogel U, Merx MW, Godecke A, Decking UKM, Schrader J (2001) Myoglobin: a scavenger of bioactive NO. PNAS 98:735–740
Flores EA, Bistrian BR, Pomposelli JJ, Dinarello CA, Blackburn GL, Istfan NW (1989) Infusion of tumor necrosis factor cachectin promotes muscle catabolism in the rat—a synergistic effect with interleukin-1. J Clin Investig 83:1614–1622
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517
Fluck M, Carson JA, Gordon SE, Ziemiecki A, Booth FW (1999) Focal adhesion proteins FAK and paxillin increase in hypertrophied skeletal muscle. Am J Physiol Cell Physiol 277:C152–C162
Franchini KG, Torsoni AS, Soares PHA, Saad MJA (2000) Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res 87:558–565
Frandsen U, LopezFigueroa M, Hellsten Y (1996) Localization of nitric oxide synthase in human skeletal muscle. Biochem Biophys Res Comm 227:88–93
Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V (2008) Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev Cell 14:661–673
Garnier A, Fortin D, Zoll J, N’Guessan B, Mettauer B, Lampert E, Veksler V, Ventura-Clapier R (2005) Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J 19:43–52
Garrido C, Galluzzi L, Brunet M, Puig PE, Didelot C, Kroemer G (2006) Mechanisms of cytochrome c release from mitochondria. Cell Death Different 13:1423–1433
Ghafourifar P, Cadenas E (2005) Mitochondrial nitric oxide synthase. Tr Pharmacol Sci 26:190–195
Gilde AJ, Van Bilsen M (2003) Peroxisome proliferator-activated receptors (PPARS): regulators of gene expression in heart and skeletal muscle. Acta Phys Scand 178:425–434
Gineitis D, Treisman R (2001) Differential usage of signal transduction pathways defines two types of serum response factor target gene. J Biol Chem 276:24531–24539
Glass DJ (2003) Molecular mechanisms modulating muscle mass. Tr Mol Med 9:344–350
Glass DJ (2005) Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984
Goldberg AL (1967) Protein synthesis in tonic and phasic skeletal muscles. Nature 216:1219–1220
Goldspink G (2005) Mechanical signals, IGF-I gene splicing, and muscle adaptation. Physiology 20:232–238
Goldspink DF, Morton AJ, Loughna P, Goldspink G (1986) The effect of hypokinesia and hypodynamia on protein-turnover and the growth of 4 skeletal-muscles of the Rat. Pflugers Arch Eur J Physiol 407:333–340
Goll DE, Thompson VF, Li HQ, Wei W, Cong JY (2003) The calpain system. Physiol Rev 83:731–801
Gordon SE, Fluck M, Booth FW (2001) Plasticity in skeletal, cardiac, and smooth muscle—selected contribution: skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol 90:1174–1183
Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N (2001) Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Gen Dev 15:1406–1418
Green H, Goreham C, Ouyang J, Ball-Burnett M, Ranney D (1999) Regulation of fiber size, oxidative potential, and capillarization in human muscle by resistance exercise. Am J Physiol-Reg Integr Comp Physiol 276:R591–R596
Gregory CM, Vandenborne K, Dudley GA (2001) Metabolic enzymes and phenotypic expression among human locomotor muscles. Muscle Nerve 24:387–393
Greig CA, Hameed M, Young A, Goldspink G, Noble B (2006) Skeletal muscle IGF-I isoform expression in healthy women after isometric exercise. Growth Hormone Igf Res 16:373–376
Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G (2008) Amino acids activate mTOR Complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab 7:456–465
Habets P, Franco D, Ruijter JM, Sargeant AJ, Pereira J, Moorman AFM (1999) RNA content differs in slow and fast muscle fibers: implications for interpretation of changes in muscle gene expression. J Histochem Cytochem 47:995–1004
Haddad F, Adams GR (2004) Inhibition of MAP/ERK kinase prevents IGF-I-induced hypertrophy in rat muscles. J Appl Physiol 96:203–210
Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N (2005) Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem 280:32081–32089
Hardie DG, Sakamoto K (2006) AMPK: A key sensor of fuel and energy status in skeletal muscle. Physiology 21:48–60
Hardy KM, Dillaman RM, Locke BR, Kinsey ST (2009) A skeletal muscle model of extreme hypertrophic growth reveals the influence of diffusion on cellular design. Am J Physiol-Reg Integr Comp Physiol 296:R1855–R1867
Hazarika S, Angelo M, Li YJ, Aldrich AJ, Odronic SI, Yan Z, Stamler JS, Annex BH (2008) Myocyte specific overexpression of myoglobin impairs angiogenesis after hind-limb ischemia. Arterioscler Thromb Vasc Biol 28:2144–2150
Heinemeier KM, Olesen JL, Schjerling P, Haddad F, Langberg H, Baldwin KM, Kjaer M (2007) Short-term strength training and the expression of myostatin and IGF-I isoforms in rat muscle and tendon: differential effects of specific contraction types. J Appl Physiol 102:573–581
Herrero A, Barja G (1997) ADP-regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: Implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr 29:241–249
Hickson RC (1980) Interference of strength development by simultaneously training for strength and endurance. Eur J Appl Physiol Occup Physiol 45:255–263
Hickson RC, Rosenkoetter MA (1981) Separate Turnover of Cytochrome-C and Myoglobin in the Red Types of Skeletal-Muscle. Am J Physiol 241:C140–C144
Hill AV (1965) Trials and trails in physiology. Edward Arnold, London
Hirota K, Semenza GL (2006) Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol 59:15–26
Hock MB, Kralli A (2009) Transcriptional Control of Mitochondrial Biogenesis and Function. Ann Rev Physiol 71:177–203
Hood DA (2001) Plasticity in skeletal, cardiac, and smooth muscle—invited review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol 90:1137–1157
Hood DA, Terjung RL (1987) Leucine metabolism in perfused rat skeletal-muscle during contractions. Am J Physiol 253:E636–E647
Hoppeler H, Billeter R (1991) conditions for oxygen and substrate transport in muscles in exercising mammals. J Exp Biol 160:263–283
Hornberger TA, Hunter RB, Kandarian SC, Esser KA (2001) Regulation of translation factors during hindlimb unloading and denervation of skeletal muscle in rats. Am J Physiol Cell Physiol 281:C179–C187
Hornberger TA, Chu WK, Mak YW, Hsiung JW, Huang SA, Chien S (2006) The role of phospholipase D and phosphatidic acid in the mechanical activation of mTOR signaling in skeletal muscle. PNAS 103:4741–4746
Hornberger TA, Sukhija KB, Wang XR, Chien S (2007) mTOR is the rapamycin-sensitive kinase that confers mechanically-induced phosphorylation of the hydrophobic motif site Thr(389) in p70(S6 k). FEBS Lett 581:4562–4566
Hunter RB, Stevenson EJ, Koncarevic A, Mitchell-Felton H, Essig DA, Kandarian SC (2002) Activation of an alternative NF-kappa B pathway in skeletal muscle during disuse atrophy. FASEB J 16:529–538
Ianuzzo CD, Blank S, Crassweller A, Spalding J, Hamilton N, Dabrowski B, Rooks N (1989) Effect of hindlimb immobilization and recovery on compensatory hypertrophied rat plantaris muscle. Mol Cell Biochem 90:57–68
Inoki K, Zhu TQ, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Jackman RW, Kandarian SC (2004) The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 287:C834–C843
Jackson MJ, Pye D, Palomero J (2007) The production of reactive oxygen and nitrogen species by skeletal muscle. J Appl Physiol 102:1664–1670
Jaspers RT (2004) Effects of strain on contractile force and number of sarcomeres in series of Xenopus laevis single muscle fibres during long-term culture. J Muscle Res Cell Motil 25:285–296
Jaspers RT, Feenstra HM, van Beek-Harmsen BJ, Huijing PA, van der Laarse WJ (2006) Differential effects of muscle fibre length and insulin on muscle-specific mRNA content in isolated mature muscle fibres during long-term culture. Cell Tissue Res 326:795–808
Jaspers RT, van Beek-Harmsen BJ, Blankenstein MA, Goldspink G, Huijing PA, van der Laarse WJ (2008) Hypertrophy of mature Xenopus muscle fibres in culture induced by synergy of albumin and insulin. Pflugers Arch Eur J Physiol 457:161–170
Jefferson LS, Fabian JR, Kimball SR (1999) Glycogen synthase kinase-3 is the predominant insulin-regulated eukaryotic initiation factor 2B kinase in skeletal muscle. Int J Biochem Cell Biol 31:191–200
Jensen TE, Wojtaszewski JFP, Richter EA (2009) AMP-activated protein kinase in contraction regulation of skeletal muscle metabolism: necessary and/or sufficient? Acta Phys 196:155–174
Jung JE, Lee JW, Ha JH, Kim SS, Cho YH, Baik HH, Kang IS (2004) 5-Aminoimidazole-4-carboxamide-ribonucleoside enhances oxidative stress-induced apoptosis through activation of nuclear factor-kappa B in mouse neuro 2a neuroblastoma cells. Neurosci Lett 354:197–200
Kamei Y, Miura S, Suzuki M, Kai Y, Mizukami J, Taniguchi T, Mochida K, Hata T, Matsuda J, Aburatani H, Nishino I, Ezaki O (2004) Skeletal muscle FOXO1 (FKHR) transgenic mice have less skeletal muscle mass, down-regulated type I (slow twitch/red muscle) fiber genes, and impaired glycemic control. J Biol Chem 279:41114–41123
Kanatous SB, Mammen PPA, Rosenberg PB, Martin CM, White MD, DiMaio JM, Huang G, Muallem S, Garry DJ (2009) Hypoxia reprograms calcium signaling and regulates myoglobin expression. Am J Physiol Cell Physiol 296:C393–C402
Kayar SR, Banchero N (1987) Volume density and distribution of mitochondria in myocardial growth and hypertrophy. Respir Physiol 70:275–286
Kimura H, Sugaya K, Cook PR (2002) The transcription cycle of RNA polymerase II in living cells. J Cell Biol 159:777–782
Kinsey ST, Hardy KM, Locke BR (2007) The long and winding road: influences of intracellular metabolite diffusion on cellular organization and metabolism in skeletal muscle. J Exp Biol 210:3505–3512
Klee CB, Ren H, Wang XT (1998) Regulation of the calmodulin-stimulated protein phosphatase, calcineurin. J Biol Chem 273:13367–13370
Klossner S, Durieux AC, Freyssenet D, Flueck M (2009) Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol 106:389–398
Kobzik L, Reid MB, Bredt DS, Stamler JS (1994) Nitric-oxide in skeletal-muscle. Nature 372:546–548
Koh TJ, Tidball JG (1999) Nitric oxide synthase inhibitors reduce sarcomere addition in rat skeletal muscle. J Physiol 519:189–196
Kominami E, Tsukahara T, Bando Y, Katunuma N (1985) Distribution of cathepsins-B and cathepsins-H in rat-tissues and peripheral-blood cells. J Biochem 98:87–93
Koopman R, Zorenc AHG, Gransier RJJ, Cameron-Smith D, van Loon LJC (2006) Increase in S6K1 phosphorylation in human skeletal muscle following resistance exercise occurs mainly in type II muscle fibers. Am J Physiol Endocrinol Metab 290:E1245–E1252
Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL (1992) Cell-adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem 267:23439–23442
Koulmann N, Bigard A (2006) Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch Eur J Physiol 452:125–139
Krawiec BJ, Nystrom GJ, Frost RA, Jefferson LS, Lang CH (2007) AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am J Physiol Endocrinol Metab 292:E1555–E1567
Kreutzer U, Jue T (2004) Role of myoglobin as a scavenger of cellular NO in myocardium. Am J Physiol Heart Circ Physiol 286:H985–H991
Kupa EJ, Roy SH, Kandarian SC, Deluca CJ (1995) Effects of muscle-fiber type and size on Emg median frequency and conduction-velocity. J Appl Physiol 79:23–32
Lagord C, Soulet L, Bonavaud S, Bassaglia Y, Rey C, Barlovatz-Meimon G, Gautron J, Martelly I (1998) Differential myogenicity of satellite cells isolated from extensor digitorum longus (EDL) and soleus rat muscles revealed in vitro. Cell Tissue Res 291:455–468
Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, Bailey J, Price SR, Mitch WE, Goldberg AL (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J 18:39–51
Lee SJ, McPherron AC (2001) Regulation of myostatin activity and muscle growth. PNAS 98:9306–9311
Lee WJ, Kim M, Park HS, Kim HS, Jeon MJ, Oh KS, Koh EH, Won JC, Kim MS, Oh GT, Yoon M, Lee KU, Park JY (2006) AMPK activation increases fatty acid oxidation in skeletal muscle by activating PPAR alpha and PGC-1. Biochem Biophys Res Commun 340:291–295
Leeuwenburgh C, Hollander J, Leichtweis S, Griffiths N, Gore M, Ji LL (1997) Adaptations of glutathione antioxidant system to endurance training are tissue and muscle fiber specific. Am J Physiol Reg Integr Comp Physiol 272:R363–R369
Lee-Young RS, Canny BJ, Myers DE, McConell GK (2009) AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol 107:283–289
Lelliott CJ, Medina-Gomez G, Petrovic N, Kis A, Feldmann HM, Bjursell M, Parker N, Curtis K, Campbell M, Hu P, Zhang DF, Litwin SE, Zaha VG, Fountain KT, Boudina S, Jimenez-Linan M, Blount M, Lopez M, Meirhaeghe A, Bohlooly-Y M, Storlien L, Stromstedt M, Snaith M, Oresic M, Abel ED, Cannon B, Vidal-Puig A (2006) Ablation of PGC-1 beta results in defective mitochondrial activity, thermogenesis, hepatic function, and cardiac performance. Plos Biol 4:2042–2056
Li YP, Chen YL, John J, Moylan J, Jin BW, Mann DL, Reid MB (2005) TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J 19:362–370
Li HL, Yin R, Chen DD, Liu D, Wang D, Yang QL, Dong YG (2007) Long-term activation of adenosine monophosphate-activated protein kinase attenuates pressure-overload-induced cardiac hypertrophy. J Cell Biochem 100:1086–1099
Lin J, Wu H, Tarr PT, Zhang CY, Wu ZD, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM (2002) Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature 418:797–801
Ling PR, Schwartz JH, Bistrian BR (1997) Mechanisms of host wasting induced by administration of cytokines in rats. Am J Physiol-Endocrinol Metab 35:E333–E339
Louis E, Raue U, Yang YF, Jemiolo B, Trappe S (2007) Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol 103:1744–1751
Machida S, Booth FW (2004) Increased nuclear proteins in muscle satellite cells in aged animals as compared to young growing animals. Exp Gerontol 39:1521–1525
MacKenzie MG, Hamilton DL, Murray JT, Taylor PM, Baar K (2009) mVps34 is activated following high-resistance contractions. J Physiol 587:253–260
Malinska D, Kudin AR, Debska-Vielhaber G, Vielhaber S, Kunz WS (2009) Quantification of superoxide production by mouse brain and skeletal muscle mitochondria. Methods Enzymol 456:419–437
Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC (2002) Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-Kinase/Akt pathway. Mol Cell 10:151–162
Mascher H, Tannerstedt J, Brink-Elfegoun T, Ekblom B, Gustafsson T, Blomstrand E (2008) Repeated resistance exercise training induces different changes in mRNA expression of MAFbx and MuRF-1 in human skeletal muscle. Am J Physiol Endocrinol Metab 294:E43–E51
Mayer RJ (2000) The meteoric rise of regulated intracellular proteolysis. Nat Rev Mol Cell Biol 1:145–148
McArdle F, Pattwell DM, Vasilaki A, McArdle A, Jackson MJ (2005) Intracellular generation of reactive oxygen species by contracting skeletal muscle cells. Free Radic Biol Med 39:651–657
McCarthy JJ, Esser KA (2007a) Counterpoint: satellite cell addition is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1100–1102
McCarthy JJ, Esser KA (2007b) MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J Appl Physiol 102:306–313
McConell GK, Bradley SJ, Stephens TJ, Canny BJ, Kingwell BA, Lee-Young RS (2007) Skeletal muscle nNOS mu protein content is increased by exercise training in humans. Am J Physiol Reg Integr Comp Physiol 293:R821–R828
McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R (2003) Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol 162:1135–1147
McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N, Smith H, Sharma M, Kambadur R (2006) Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappa B-independent, FoxO1-dependent mechanism. J Cell Physiol 209:501–514
McKoy G, Ashley W, Mander J, Yang SY, Williams N, Russell B, Goldspink G (1999) Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J Physiol 516:583–592
Mendias CL, Marcin JE, Calerdon DR, Faulkner JA (2006) Contractile properties of EDL and soleus muscles of myostatin-deficient mice. J Appl Physiol 101:898–905
Miano JM, Long XC, Fujiwara K (2007) Serum response factor: master regulator of the actin cytoskeleton and contractile apparatus. Am J Physiol Cell Physiol 292:C70–C81
Michel RN, Chin ER, Chakkalakal JV, Eibl JK, Jasmin BJ (2007) Ca2+/calmodulin-based signalling in the regulation of the muscle fibre phenotype and its therapeutic potential via modulation of utrophin A and myostatin expression. Appl Physiol Nutr Metabol 32:921–929
Millward DJ, Garlick PJ, James WPT, Nnanyelu Do, Ryatt JS (1973) Relationship between protein-synthesis and RNA content in skeletal-muscle. Nature 241:204–205
Mitchell PO, Mills ST, Pavlath GK (2002) Calcineurin differentially regulates maintenance and growth of phenotypically distinct muscles. Am J Physiol Cell Physiol 282:C984–C992
Molnar AM, Servais S, Guichardant M, Lagarde M, Macedo DV, Pereira-Da-Silva L, Sibille B, Favier R (2006) Mitochondrial H2O2 production is reduced with acute and chronic eccentric exercise in rat skeletal muscle. Antioxid Redox Signal 8:548–558
Moss FP, Leblond CP (1971) Satellite cells as source of nuclei in muscles of growing rats. Anat Rec 170:421–435
Mounier R, Lantier L, Leclerc J, Sotiropoulos A, Pende M, Daegelen D, Sakamoto K, Foretz M, Viollet B (2009) Important role for AMPK alpha 1 in limiting skeletal muscle cell hypertrophy. FASEB J 23:2264–2273
Murgia M, Serrano AL, Calabria E, Pallafacchina G, Lomo T, Schiaffino S (2000) Ras is involved in nerve-activity-dependent regulation of muscle genes. Nat Cell Biol 2:142–147
Murphy MP (2009) How mitochondria produce reactive oxygen species. Biochem J 417:1–13
Musaro A, McCullagh KJA, Naya FJ, Olson EN, Rosenthal N (1999) IGF-1 induces skeletal myocyte hypertrophy through calcineurin in association with GATA-2 and NF-ATc1. Nature 400:581–585
Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N (2001) Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet 27:195–200
Nader GA, Esser KA (2001) Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90:1936–1942
Nakatani T, Nakashima T, Kita T, Hirofuji C, Itoh K, Itoh M, Ishihara A (1999) Succinate dehydrogenase activities of fibers in the rat extensor digitorum longus, soleus, and cardiac muscles. Arch Histol Cytol 62:393–399
Narkar VA, Downes M, Yu RT, Embler E, Wang YX, Banayo E, Mihaylova MM, Nelson MC, Zou Y, Juguilon H, Kang H, Shaw RJ, Evans RM (2008) AMPK and PPARdelta agonists are exercise mimetics. Cell 134:1–11
Nathan C, Xie QW (1994) Nitric-Oxide Synthases - Roles, Tolls, and Controls. Cell 78:915–918
Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN (2000) Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem 275:4545–4548
Nguyen HX, Tidball JG (2003) Expression of a muscle-specific, nitric oxide synthase transgene prevents muscle membrane injury and reduces muscle inflammation during modified muscle use in mice. J Physiol 550:347–356
Ni YG, Berenji K, Wang N, Oh M, Sachan N, Dey A, Cheng J, Lu GR, Morris DJ, Castrillon DH, Gerard RD, Rothermel BA, Hill JA (2006) Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 114:1159–1168
Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO (2003) Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science 299:896–899
Nordquist J, Hoeglund AS, Norman H, Tang X, Dworkin B, Larsson L (2007) Transcription factors in muscle atrophy caused by blocked neuromuscular transmission and muscle unloading in rats. Mol Med 13:461–470
O’Connor RS, Pavlath GK (2007) Point: Counterpoint: Satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol 103:1099–1100
Okayasu T, Tomizawa A, Suzuki K, Manaka KI, Hattori Y (2008) PPAR alpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappa B activation through AMP-activated protein kinase activation. Life Sci 82:884–891
Paddon-Jones D, Rasmussen BB (2009) Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care 12:86–90
Perkins ND (2000) The Rel/NF-kappa B family: friend and foe. Tr Biochem Sci 25:434–440
Petrella JK, Kim JS, Cross JM, Kosek DJ, Bamman MM (2006) Efficacy of myonuclear addition may explain differential myofiber growth among resistance-trained young and older men and women. Am J Physiol Endocrinol Metab 291:E937–E946
Pette D, Staron RS (2001) Transitions of muscle fiber phenotypic profiles. Histochem Cell Biol 115:359–372
Pfeifer M, Begerow B, Minne HW (2002) Vitamin D and muscle function. Osteopor Int 13:187–194
Phillips T, Leeuwenburgh C (2005) Muscle fiber-specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J 19:668–670
Plomgaard P, Penkowa M, Pedersen BK (2005) Fiber type specific expression of TNF-alpha, IL-6 an IL-18 in human skeletal muscles. Exerc Immunol Rev 11:53–63
Plomgaard P, Penkowa M, Leick L, Pedersen BK, Saltin B, Pilegaard H (2006) The mRNA expression profile of metabolic genes relative to MHC isoform pattern in human skeletal muscles. J Appl Physiol 101:817–825
Potter CJ, Pedraza LG, Xu T (2002) Akt regulates growth by directly phosphorylating Tsc2. Nat Cell Biol 4:658–665
Powers SK, Ji LL, Leeuwenburgh C (1999) Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc 31:987–997
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol 102:2389–2397
Primeau AJ, Adhihetty PJ, Hood DA (2002) Apoptosis in heart and skeletal muscle. Can J Appl Physiol 27:349–395
Rando TA (2001) The dystrophin–glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve 24:1575–1594
Razeghi P, Buksinska-Lisik M, Palanichamy N, Stepkowski S, Frazier OH, Taegtmeyer H (2006) Transcriptional regulators of ribosomal biogenesis are increased in the unloaded heart. FASEB J 20:1090–1096
Reichmann H, Wasl R, Simoneau JA, Pette D (1991) Enzyme-activities of fatty-acid oxidation and the respiratory-chain in chronically stimulated fast-twitch muscle of the rabbit. Pflugers Arch Eur J Physiol 418:572–574
Reid WD, MacGowan NA (1998) Respiratory muscle injury in animal models and humans. Mol Cell Biochem 179:63–80
Richardson RS, Duteil S, Wary C, Wray DW, Hoff J, Carlier PG (2006) Human skeletal muscle intracellular oxygenation: the impact of ambient oxygen availability. J Physiol 571:415–424
Rivero JLL, Talmadge RJ, Edgerton VR (1999) Interrelationships of myofibrillar ATPase activity and metabolic properties of myosin heavy chain-based fibre types in rat skeletal muscle. Histochem Cell Biol 111:277–287
Roghani M, Mansukhani A, Dellera P, Bellosta P, Basilico C, Rifkin DB, Moscatelli D (1994) Heparin increases the affinity of basic fibroblast growth-factor for its receptor but is not required for binding. J Biol Chem 269:3976–3984
Rossi-Fanelli A, Antonini E (1958) Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys 77:478–492
Russell ST, Eley HL, Wyke SM, Tisdale MJ (2008) Involvement of phosphoinositide 3-kinase and Akt in the induction of muscle protein degradation by proteolysis-inducing factor. Biochem J 409:751–759
Ruwhof C, van der Laarse A (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47:23–37
Sadoshima J, Izumo S (1993) Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes—potential involvement of an autocrine paracrine mechanism. EMBO J 12:1681–1692
Sáinz N, Rodríguez A, Catalán V, Becerril S, Ramírez B, Gómez-Ambrosi J, Frühbeck G (2009) Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS ONE 4:e6808
Sakamoto K, Hirshman MF, Aschenbach WG, Goodyear LJ (2002) Contraction regulation of Akt in rat skeletal muscle. J Biol Chem 277:11910–11917
Sakuma K, Watanabe K, Hotta N, Koike T, Ishida K, Katayama K, Akima H (2009) The adaptive responses in several mediators linked with hypertrophy and atrophy of skeletal muscle after lower limb unloading in humans. Acta Physiol (Oxf) [Epub ahead of print]
Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL (2004) Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117:399–412
Sandri M, Lin JD, Handschin C, Yang WL, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM (2006) PGC-1 alpha a protects skeletal muscle from atrophy by suppressing Fox03 action and atrophy-specific gene transcription. PNAS 103:16260–16265
Sayegh JF, Lajtha A (1989) In vivo rates of protein synthesis in brain, muscle, and liver of 5 vertebrate species. Neurochem Res 14:1165–1168
Schisler JC (2008) You spin me round—MAFbx/Atrogin-1 feeds forward on FOXO transcription factors (like a record). Cell Cycle 7:440–443
Schmalbruch H, Hellhammer U (1977) Number of nuclei in adult rat muscles with special reference to satellite cells. Anat Rec 189:169–175
Schulman H (1993) The multifunctional Ca2+/calmodulin-dependent protein kinases. Curr Opin Cell Biol 5:247–253
Schultz E, Chamberlain C, McCormick KM, Mozdziak PE (2006) Satellite cells express distinct patterns of myogenic proteins in immature skeletal muscle. Dev Dyn 235:3230–3239
Sempere LF, Freemantle S, Pitha-Rowe I, Moss E, Dmitrovsky E, Ambros V (2004) Expression profiling of mammalian microRNAs uncovers a subset of brain-expressed microRNAs with possible roles in murine and human neuronal differentiation. Genome Biol 5:R13
Semsarian C, Wu MJ, Ju YK, Marciniec T, Yeoh T, Allen DG, Harvey RP, Graham RM (1999) Skeletal muscle hypertrophy is mediated by a Ca2+-dependent calcineurin signalling pathway. Nature 400:576–581
Serrano AL, Murgia M, Pallafacchina G, Calabria E, Coniglio P, Lomo T, Schiaffino S (2001) Calcineurin controls nerve activity-dependent specification of slow skeletal muscle fibers but not muscle growth. PNAS 98:13108–13113
Shi H, Scheffler JM, Zeng CY, Pleitner JM, Hannon KM, Grant AL, Gerrard DE (2009) Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol 296:C1040–C1048
Shimizu M, Webster C, Morgan DO, Blau HM, Roth RA (1986) Insulin and insulin-like growth-factor receptors and responses in cultured human-muscle cells. Am J Physiol 251:E611–E615
Simoneau JA, Bouchard C (1989) Human variation in skeletal-muscle fiber-type proportion and enzyme-activities. Am J Physiol 257:E567–E572
Smith RS, Ovalle WK (1973) Varieties of fast and slow extrafusal muscle-fibers in amphibian hind limb muscles. J Anat 116:1–24
Song XM, Ryder JW, Kawano Y, Chibalin AV, Krook A, Zierath JR (1999) Muscle fiber type specificity in insulin signal transduction. Am J Physiol Reg Integr Comp Physiol 277:R1690–R1696
Southgate RJ, Neill B, Prelovsek O, El-Osta A, Kamei Y, Miura S, Ezaki O, McLoughlin TJ, Zhang W, Unterman TG, Febbraio MA (2007) FOXO1 regulates the expression of 4E-BP1 and inhibits mTOR signaling in mammalian skeletal muscle. J Biol Chem 282:21176–21186
Spangenburg EE (2009) Changes in muscle mass with mechanical load: possible cellular mechanisms. Appl Physiol Nutr Metab 34:328–335
Spangenburg EE, McBride TA (2006) Inhibition of stretch-activated channels during eccentric muscle contraction attenuates p70(S6K) activation. J Appl Physiol 100:129–135
Spangenburg EE, Le Roith D, Ward CW, Bodine SC (2008) A functional insulin-like growth factor receptor is not necessary for load-induced skeletal muscle hypertrophy. J Physiol 586:283–291
Stewart C, Pell JM, Flueck M, Goldspink G (2009) Point: Counterpoint “IGF is/is not the major physiological regulator of muscle mass. J Appl Physiol [Epub ahead of print]
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ (2004) The IGF-1/PI3K/Akt pathway prevents short article expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14:395–403
St-Pierre J, Buckingham JA, Roebuck SJ, Brand MD (2002) Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem 277:44784–44790
Taillandier D, Aurousseau E, MeynialDenis D, Bechet D, Ferrara M, Cottin P, Ducastaing A, Bigard X, Guezennec CY, Schmid HP, Attaix D (1996) Coordinate activation of lysosomal, Ca2+-activated and ATP-ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem J 316:65–72
Terjung RL (1979) Turnover of cytochrome-C in different skeletal-muscle fiber types of the rat. Biochem J 178:569–574
Thomson DM, Fick CA, Gordon SE (2008) AMPK activation attenuates S6K1, 4E-BP1, and eEF2 signaling responses to high-frequency electrically stimulated skeletal muscle contractions. J Appl Physiol 104:625–632
Torrejais MM, Soares JC, Matheus SMM, Cassel FD, Curi PR (1999) Histochemical study of the extensor digitorum longus and soleus muscles in alcoholic rats. Anat Histol Embryol J Vet Med Ser C 28:367–373
Toth MJ, Tchernof A (2006) Effect of age on skeletal muscle myofibrillar mRNA abundance: relationship to myosin heavy chain protein synthesis rate. Exp Gerontol 41:1195–1200
Trendelenburg AU, Meyer A, Rohner D, Boyle J, Hatakeyama S, Glass DJ (2009) Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol 296:C1258–C1270
Tseng BS, Kasper CE, Edgerton VR (1994) Cytoplasm-to-myonucleus ratios and succinate-dehydrogenase activities in adult-rat slow and fast muscle-fibers. Cell Tissue Res 275:39–49
van Beek-Harmsen BJ, van der Laarse WJ (2005) Immunohistochemical determination of cytosolic cytochrome c concentration in cardiomyocytes. J Histochem Cytochem 53:803–807
van Beek-Harmsen BJ, Bekedam MA, Feenstra HM, Visser FC, van der Laarse WJ (2004) Determination of myoglobin concentration and oxidative capacity in cryostat sections of human and rat skeletal muscle fibres and rat cardiomyocytes. Histochem Cell Biol 121:335–342
van der Laarse WJ, Diegenbach PC, Elzinga G (1989) Maximum rate of oxygen consumption and quantitative histochemistry of succinate dehydrogenase in single muscle fibres of Xenopus laevis. J Muscle Res Cell Motil 10:221–228
Van Der Laarse WJ, Des Tombe AL, Lee-de Groot MBE, Diegenbach PC (1998) Size principle of striated muscle cells. Neth J Zool 48:213–223
van der Laarse WJ, des Tombe AL, van Beek-Harmsen BJ, Lee-de Groot MBE, Jaspers RT (2005) Krogh’s diffusion coefficient for oxygen in isolated Xenopus skeletal muscle fibers and rat myocardial trabeculae at maximum rates of oxygen consumption. J Appl Physiol 99:2173–2180
van Weel V, Deckers MML, Grimbergen JM, van Leuven KJM, Lardenoye J, Schlingemann RO, Amerongen GPV, van Bockel JH, van Hinsbergh VWM, Quax PHA (2004) Vascular endothelial growth factor overexpression in ischemic skeletal muscle enhances myoglobin expression in vivo. Circ Res 95:58–66
Vasilaki A, Csete M, Pye D, Lee S, Palomero J, McArdle F, Van Rernmen H, Richardson A, McArdle A, Faulkner JA, Jackson MJ (2006) Genetic modification of the manganese superoxide dismutase/glutathione peroxidase 1 pathway influences intracellular ROS generation in quiescent, but not contracting, skeletal muscle cells. Free Radic Biol Med 41:1719–1725
Vescovo G, Dalla Libera L (2006) Skeletal muscle apoptosis in experimental heart failure: the only link between inflammation and skeletal muscle wastage? Curr Opin Clin Nutr Metab Care 9:416–422
Wang N, Butler JP, Ingber DE (1993) Mechanotransduction across the cell-surface and through the cytoskeleton. Science 260:1124–1127
Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, Schwartz RJ (1998) RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem 273:30287–30294
Wicks KL, Hood DA (1991) Mitochondrial adaptations in denervated muscle—relationship to muscle performance. Am J Physiol 260:C841–C850
Williams RS (1986) Mitochondrial gene-expression in mammalian striated-muscle—evidence that variation in gene dosage is the major regulatory event. J Biol Chem 261:2390–2394
Wretman C, Widegren U, Lionikas A, Westerblad H, Henriksson J (2000) Differential activation of mitogen-activated protein kinase signalling pathways by isometric contractions in isolated slow- and fast-twitch rat skeletal muscle. Acta Phys Scand 170:45–49
Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS (2001) Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J 20:6414–6423
Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS (2002) Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 296:349–352
Wust RC, Jaspers RT, van Heijst AF, Hopman MT, Hoofd LJ, van der Laarse WJ, Degens H (2009) Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia. Am J Physiol Heart Circ Physiol 297:H364–H374
Xia H, Nho RS, Kahm J, Kleidon J, Henke CA (2004) Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta(1) integrin viability signaling pathway. J Biol Chem 279:33024–33034
Yang SY, Goldspink GE (2002) Different roles of the IGF-I Ec peptide (MGF) and mature IGF-I in myoblast proliferation and differentiation. FEBS Lett 522:156–160
Yazaki Y, Komuro I (1992) Role of protein-kinase system in the signal transduction of stretch-mediated myocyte growth. Basic Res Cardiol 87:11–18
Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG (2005) Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol 562:367–380
Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z (2008) Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS ONE 7:e2086
Zamir O, Hasselgren PO, Vonallmen D, Fischer JE (1993) In vivo administration of interleukin-1-alpha induces muscle proteolysis in normal and adrenalectomized rats. Metabolism 42:204–208
Zanchi NE, de Siqueira MV, Lira FS, Rosa JC, Yamashita AS, Carvalho CRD, Seelaender M, Lancha AH (2009) Chronic resistance training decreases MuRF-1 and Atrogin-1 gene expression but does not modify Akt, GSK-3 beta and p70S6K levels in rats. Eur J Appl Physiol 106:415–423
Zhao M, New L, Kravchenko VV, Kato Y, Gram H, di Padova F, Olson EN, Ulevitch RJ, Han JH (1999) Regulation of the MEF2 family of transcription factors by p38. Mol Cell Biol 19:21–30
Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ (2002) Induction of cachexia in mice by systemically administered myostatin. Science 296:1486–1488
Acknowledgments
The authors are grateful to Carla Offringa and Dorien Kos for their expert assistance with the PCR experiments and in situ hybridizations. We thank Bruno Della Gaspera for kindly providing the plasmid for the actin probe and Annemieke van Dijk for providing the animals for the PCR experiments.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Nigel Taylor.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Wessel, T., de Haan, A., van der Laarse, W.J. et al. The muscle fiber type–fiber size paradox: hypertrophy or oxidative metabolism?. Eur J Appl Physiol 110, 665–694 (2010). https://doi.org/10.1007/s00421-010-1545-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00421-010-1545-0