Abstract
Purpose
In vivo microenvironments are critical to tissue homeostasis and wound healing, and the cornea is regulated by a specific microenvironment complex that consists of cell–cell interactions, air–liquid interfaces, and fluid flow stimulation. In this study, we aimed to clarify the effects of and the correlations among these three component factors on the cell kinetics of corneal epithelial cells.
Methods
Human corneal epithelial–transformed (HCE–T) cells were cocultured with either primary rat corneal fibroblasts or NIH 3T3 fibroblasts. We employed a double-dish culture method to create an air–liquid interface and a gyratory shaker to create fluid flow stimulation. Morphometric and protein expression analyses were performed for the HCE–T cells.
Results
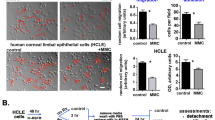
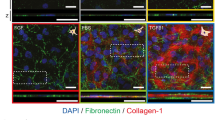
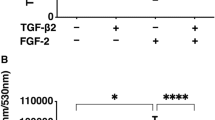
Both the primary rat fibroblasts and the NIH 3T3 cells promoted HCE–T cell proliferation, and the presence of fluid flow synergistically enhanced this effect and inhibited the apoptosis of HCE–T cells. Moreover, fluid flow enhanced the emergence of myofibroblasts when cocultured with primary rat fibroblasts or NIH 3T3 cells. Extracellular signal-regulated kinase and p38 signaling were regulated either synergistically or independently by both fluid flow and cellular interaction between the HCE–T and NIH 3T3 cells.
Conclusion
The cell–cell interaction and fluid flow stimulation in the air–liquid interface synergistically or independently regulated the behavior of HCE–T cells. Fluid flow accelerated the phenotypic change from corneal fibroblasts and NIH 3T3 cells to myofibroblasts. Elucidation of the multicomponent interplay in this microenvironment will be critical to the homeostasis and regeneration of the cornea and other ocular tissues.




Similar content being viewed by others
References
Pawlina W, Ross MH (2018) Histology: a text and atlas: with correlated cell and molecular biology. Wolters Kluwer Health, Philadelphia
Wilson SE, Mohan RR, Mohan RR, Ambrosio R Jr, Hong J, Lee J (2001) The corneal wound healing response:: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res 20(5):625–637
Zhang X, Vimalin Jeyalatha M, Qu Y, He X, Ou S, Bu J, Jia C, Wang J, Wu H, Liu Z, Li W (2017) Dry eye management: targeting the ocular surface microenvironment. Int J Mol Sci 18(7). https://doi.org/10.3390/ijms18071398
Joyce N (2003) Proliferative capacity of the corneal endothelium. Prog Retin Eye Res 22(3):359–389. https://doi.org/10.1016/s1350-9462(02)00065-4
Blanpain C, Fuchs E (2009) Epidermal homeostasis: a balancing act of stem cells in the skin. Nat Rev Mol Cell Biol 10(3):207
Diegelmann RF, Evans MC (2004) Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 9(1):283–289
Joyce JA, Pollard JW (2009) Microenvironmental regulation of metastasis. Nat Rev Cancer 9(4):239
Walker GM, Zeringue HC, Beebe DJ (2004) Microenvironment design considerations for cellular scale studies. Lab Chip 4(2):91–97
Fernandez-Perez J, Ahearne M (2019) Influence of biochemical cues in human corneal stromal cell phenotype. Curr Eye Res 44(2):135–146. https://doi.org/10.1080/02713683.2018.1536216
Minami Y, Sugihara H, Oono S (1993) Reconstruction of cornea in three-dimensional collagen gel matrix culture. Invest Ophthalmol Vis Sci 34(7):2316–2324
Nishimura T, Toda S, Mitsumoto T, Oono S, Sugihara H (1998) Effects of hepatocyte growth factor, transforming growth factor-beta1 and epidermal growth factor on bovine corneal epithelial cells under epithelial-keratocyte interaction in reconstruction culture. Exp Eye Res 66(1):105–116. https://doi.org/10.1006/exer.1997.0419
Aoki S, Makino J, Nagashima A, Takezawa T, Nomoto N, Uchihashi K, Matsunobu A, Sanai T, Sugihara H, Toda S (2011) Fluid flow stress affects peritoneal cell kinetics: possible pathogenesis of peritoneal fibrosis. Perit Dial Int 31(4):466–476
Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM (1996) Induction of alpha-smooth muscle actin expression and myofibroblast transformation in cultured corneal keratocytes. Cornea 15(5):505–516
Jester JV, Petroll WM, Cavanagh HD (1999) Corneal stromal wound healing in refractive surgery: the role of myofibroblasts. Prog Retin Eye Res 18(3):311–356
Kawakita T, Espana EM, He H, Li W, Liu CY, Tseng SC (2005) Intrastromal invasion by limbal epithelial cells is mediated by epithelial-mesenchymal transition activated by air exposure. Am J Pathol 167(2):381–393. https://doi.org/10.1016/S0002-9440(10)62983-5
Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE (2006) Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res 82(5):788–797
Verjans GM, Remeijer L, Mooy CM, Osterhaus AD (2000) Herpes simplex virus–specific T cells infiltrate the cornea of patients with herpetic stromal keratitis: no evidence for autoreactive T cells. Invest Ophthalmol Vis Sci 41(9):2607–2612
Wang Z, Yang H, Tachado SD, Capó-Aponte JE, Bildin VN, Koziel H, Reinach PS (2006) Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci 47(12):5267–5275
Dewey C, Bussolari S, Gimbrone M, Davies PF (1981) The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng 103(3):177–185
Davies PF (2009) Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat Rev Cardiol 6(1):16
Ahsan T, Nerem RM (2010) Fluid shear stress promotes an endothelial-like phenotype during the early differentiation of embryonic stem cells. Tissue Eng A 16(11):3547–3553
Akutagawa T, Aoki S, Yamamoto-Rikitake M, Iwakiri R, Fujimoto K, Toda S (2018) Cancer–adipose tissue interaction and fluid flow synergistically modulate cell kinetics, HER2 expression, and trastuzumab efficacy in gastric cancer. Gastric Cancer 21(6):946–955
Ren H, Wilson G (1997) The effect of a shear force on the cell shedding rate of the corneal epithelium. Acta Ophthalmol Scand 75(4):383–387
Molladavoodi S, Robichaud M, Wulff D, Gorbet M (2017) Corneal epithelial cells exposed to shear stress show altered cytoskeleton and migratory behaviour. PLoS One 12(6):e0178981
Kaji Y, Oshika T, Usui T, Sakakibara J (2005) Effect of shear stress on attachment of corneal endothelial cells in association with corneal endothelial cell loss after laser iridotomy. Cornea 24(8):S55–S58
Yamamoto Y, Uno T, Joko T, Shiraishi A, Ohashi Y (2010) Effect of anterior chamber depth on shear stress exerted on corneal endothelial cells by altered aqueous flow after laser iridotomy. Invest Ophthalmol Vis Sci 51(4):1956–1964
Aoki S, Toda S, Ando T, Sugihara H (2004) Bone marrow stromal cells, preadipocytes, and dermal fibroblasts promote epidermal regeneration in their distinctive fashions. Mol Biol Cell 15(10):4647–4657
Sugihara H, Toda S, Miyabara S, Kusaba Y, Minami Y (1991) Reconstruction of the skin in three-dimensional collagen gel matrix culture. In Vitro Cell Dev Biol 27(2):142–146
Inoue T, Toda S, Narisawa Y, Sugihara H (2001) Subcutaneous adipocytes promote the differentiation of squamous cell carcinoma cell line (DJM-1) in collagen gel matrix culture. J Investig Dermatol 117(2):244–250
Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, Sugihara H, Fujimoto K, Weissman IL, Capecchi MR (2009) Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med 15(6):701
Wilson SE, Netto M, Ambrosio R Jr (2003) Corneal cells: chatty in development, homeostasis, wound healing, and disease. Am J Ophthalmol 136(3):530–536
Kalluri R, Zeisberg M (2006) Fibroblasts in cancer. Nat Rev Cancer 6(5):392–401. https://doi.org/10.1038/nrc1877
Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN (2014) Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy 16(5):579–585. https://doi.org/10.1016/j.jcyt.2013.07.017
Dittmer J, Leyh B (2014) Paracrine effects of stem cells in wound healing and cancer progression. Int J Oncol 44(6):1789–1798
Bowling B (2015) Kanski’s clinical ophthalmology, 8th edn. Elsevier Health Sciences, London
Myrna KE, Pot SA, Murphy CJ (2009) Meet the corneal myofibroblast: the role of myofibroblast transformation in corneal wound healing and pathology. Vet Ophthalmol 12:25–27
Wilson SE (2012) Corneal myofibroblast biology and pathobiology: generation, persistence, and transparency. Exp Eye Res 99:78–88
Kawashima M, Kawakita T, Higa K, Satake Y, Omoto M, Tsubota K, Shimmura S, Shimazaki J (2010) Subepithelial corneal fibrosis partially due to epithelial-mesenchymal transition of ocular surface epithelium. Mol Vis 16:2727
Furukawa F, Matsuzaki K, Mori S, Tahashi Y, Yoshida K, Sugano Y, Yamagata H, Matsushita M, Seki T, Inagaki Y (2003) p38 MAPK mediates fibrogenic signal through Smad3 phosphorylation in rat myofibroblasts. Hepatology 38(4):879–889
Caraci F, Gili E, Calafiore M, Failla M, La Rosa C, Crimi N, Sortino MA, Nicoletti F, Copani A, Vancheri C (2008) TGF-β1 targets the GSK-3β/β-catenin pathway via ERK activation in the transition of human lung fibroblasts into myofibroblasts. Pharmacol Res 57(4):274–282
Acknowledgments
We are grateful to Professor S. Aishima and Dr. M. Hashiguchi for useful discussion and for sharing their dataset, and we thank T. Sakumoto, S. Morito, M. Nishida, F. Mutoh, S. Nakahara, and I. Nanbu for their excellent technical assistance. We also thank Mr. K. Tokaichi for refining the English of the manuscript. We also thank Enago (www.enago.jp) for the English language review.
Funding
This work was supported in part by the Center for Clinical and Translational Research of Kyushu University Hospital (to S.A.), and Grants-in-Aid from the Japanese Ministry of Education, Culture, Sports, Science and Technology for Scientific Research (no. 16K09284 to S.A. and no. 18K09451 to H.E.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments complied with the guidelines of the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kawata, K., Aoki, S., Futamata, M. et al. Mesenchymal cells and fluid flow stimulation synergistically regulate the kinetics of corneal epithelial cells at the air–liquid interface. Graefes Arch Clin Exp Ophthalmol 257, 1915–1924 (2019). https://doi.org/10.1007/s00417-019-04422-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-019-04422-y