Abstract
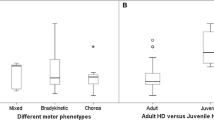
Substantia nigra (SN) hyperechogenicity can be detected by transcranial sonography (TCS) to assist the early diagnosis of idiopathic Parkinson’s disease (IPD). This study prospectively investigated whether SN hyperechogenicity is also present in Huntington’s disease (HD) patients with symptoms of hypokinesia and/or rigidity. All patients recruited to the study (n = 15) were characterised by hypokinesia and/or rigidity while nine of these patients also displayed chorea and/or dystonia. The control group included 15 individuals. Clinical examination was documented using the Unified Huntington’s Disease Rating Scale (UHDRS). TCS examination revealed SN hyperechogenicity in 14/15 (93.3 %) patients (9/14 unilateral, 5/14 bilateral). Hyperechogenicity of the caudate and lentiform nuclei (CN, LN) was less frequent (CN: 80 % total, LN: 53.3 % total). This is the first study to assess SN hyperechogenicity in hypokinetic HD patients. Assuming that the primary sites of pathology in IPD and HD are the SN and the striatum, respectively, our observations suggest a functional impairment of the nigrostriatal system in HD, an effect that is potentially independent of the primarily-affected basal ganglia nucleus.


Similar content being viewed by others
References
Walter U, Niehaus L, Probst T, Benecke R, Meyer BU, Dressler D (2003) Brain parenchyma sonography discriminates Parkinson’s disease and atypical parkinsonian syndromes. Neurology 60(1):74–77
Hellwig S, Reinhard M, Amtage F, Guschlbauer B, Buchert R, Tuscher O, Weiller C, Niesen WD, Meyer PT (2014) Transcranial sonography and [18F]fluorodeoxyglucose positron emission tomography for the differential diagnosis of parkinsonism: a head-to-head comparison. Eur J Neurol 21(6):860–866
Berg D, Godau J, Walter U (2008) Transcranial sonography in movement disorders. Lancet Neurol 7(11):1044–1055
Walter U, Kanowski M, Kaufmann J, Grossmann A, Benecke R, Niehaus L (2008) Contemporary ultrasound systems allow high-resolution transcranial imaging of small echogenic deep intracranial structures similarly as MRI: a phantom study. Neuroimage. 40(2):551–558
Sieradzan KA, Mann DM (2001) The selective vulnerability of nerve cells in Huntington’s disease. Neuropathol Appl Neurobiol 27(1):1–21
Phillips W, Shannon KM, Barker RA (2008) The current clinical management of Huntington’s disease. Mov Disord 23(11):1491–1504
Bittenbender JB, Quadfasel FA (1962) Rigid and akinetic forms of Huntington’s chorea. Arch Neurol 7:275–288
Postert T, Lack B, Kuhn W, Jergas M, Andrich J, Braun B, Przuntek H, Sprengelmeyer R, Agelink M, Buttner T (1999) Basal ganglia alterations and brain atrophy in Huntington’s disease depicted by transcranial real time sonography. J Neurol Neurosurg Psychiatry 67(4):457–462
Krogias C, Strassburger K, Eyding J, Gold R, Norra C, Juckel G, Saft C, Ninphius D (2011) Depression in patients with Huntington disease correlates with alterations of the brain stem raphe depicted by transcranial sonography. J Psychiatry Neurosci 36(3):187–194
Hsg (1996) Unified Huntington’s Disease Rating Scale: reliability and consistency. Huntington Study Group. Mov Disord 11(2):136–142
Walter U, Behnke S, Eyding J, Niehaus L, Postert T, Seidel G, Berg D (2007) Transcranial brain parenchyma sonography in movement disorders: state of the art. Ultrasound Med Biol 33(1):15–25
Berg D, Jabs B, Merschdorf U, Beckmann H, Becker G (2001) Echogenicity of substantia nigra determined by transcranial ultrasound correlates with severity of parkinsonian symptoms induced by neuroleptic therapy. Biol Psychiatry 50(6):463–467
Berardelli A, Noth J, Thompson PD, Bollen EL, Curra A, Deuschl G, Van Dijk JG, Topper R, Schwarz M, Roos RA (1999) Pathophysiology of chorea and bradykinesia in Huntington’s disease. Mov Disord 14(3):398–403
Thompson PD, Berardelli A, Rothwell JC, Day BL, Dick JP, Benecke R, Marsden CD (1988) The coexistence of bradykinesia and chorea in Huntington’s disease and its implications for theories of basal ganglia control of movement. Brain 111(Pt 2):223–244
Schweitzer KJ, Hilker R, Walter U, Burghaus L, Berg D (2006) Substantia nigra hyperechogenicity as a marker of predisposition and slower progression in Parkinson’s disease. Mov Disord 21(1):94–98
Berg D, Roggendorf W, Schroder U, Klein R, Tatschner T, Benz P, Tucha O, Preier M, Lange KW, Reiners K, Gerlach M, Becker G (2002) Echogenicity of the substantia nigra: association with increased iron content and marker for susceptibility to nigrostriatal injury. Arch Neurol 59(6):999–1005
Lumsden AL, Henshall TL, Dayan S, Lardelli MT, Richards RI (2007) Huntingtin-deficient zebrafish exhibit defects in iron utilization and development. Hum Mol Genet 16(16):1905–1920
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P, Marsden CD (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(Pt 4):1953–1975
Rosas HD, Chen YI, Doros G, Salat DH, Chen NK, Kwong KK, Bush A, Fox J, Hersch SM (2012) Alterations in brain transition metals in Huntington disease: an evolving and intricate story. Arch Neurol 69(7):887–893
Muller M, Leavitt BR (2014) Iron dysregulation in Huntington’s disease. J Neurochem 130:328–350
Jellinger KA (2013) The relevance of metals in the pathophysiology of neurodegeneration, pathological considerations. Int Rev Neurobiol 110:1–47
Jabs BE, Berg D, Merschdorf U, Bartsch AJ, Pfuhlmann B (2001) Differences in substantia nigra echogenicity of nosological subtypes within the schizophrenic spectrum. A preliminary transcranial ultrasound study. Neuropsychobiology 44(4):183–186
Behnke S, Runkel A, Kassar HA, Ortmann M, Guidez D, Dillmann U, Fassbender K, Spiegel J (2013) Long-term course of substantia nigra hyperechogenicity in Parkinson’s disease. Mov Disord 28(4):455–459
Acknowledgments
The authors wish to thank the European Huntington’s Disease network (EHDN), in particular Katrin Barth, for technical support. This study was funded by the EHDN. Further they would like to thank GE for providing the ultrasound system. The authors thank Dr. Sandra Dieni for helpful comments on the manuscript.
Conflicts of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Ethical standard
This study was performed in accordance with the ethical standards.
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Lambeck and W.-D. Niesen contributed equally.
Rights and permissions
About this article
Cite this article
Lambeck, J., Niesen, WD., Matthias, R. et al. Substantia nigra hyperechogenicity in hypokinetic Huntington’s disease patients. J Neurol 262, 711–717 (2015). https://doi.org/10.1007/s00415-014-7587-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-014-7587-1