Abstract
Density measurements on nine liquids in the CaCO3–Li2CO3–Na2CO3–K2CO3 quaternary system were performed at 1 bar between 555 and 969 °C using the double-bob Archimedean method. Our density data on the end-member alkali carbonate liquids are in excellent agreement with the NIST standards compiled by Janz (1992). The results were fitted to a volume equation that is linear in composition and temperature; this model recovers the measured volumes within experimental error (±0.18% on average, with a maximum residual of ±0.50%). Our results indicate that the density of the CaCO3 component in natrocarbonate liquids is 2.502 (±0.014) g/cm3 at 800 °C and 1 bar, which is within the range of silicate melts; its coefficient of thermal expansion is 1.8 (±0.5)×10−4 K−1 at 800 °C. Although the volumes of carbonate liquids mix linearly with respect to carbonate components, they do not mix linearly with silicate liquids. Our data are used with those in the literature to estimate the value of \( \overline V _{CO_2 } \) in alkaline silicate magmas (≥20 cm3/mol at 1400 °C and 20 kbar), where CO2 is dissolved as carbonate in close association with Ca. Our volume measurements are combined with sound speed data in the literature to derive the compressibility of the end-member liquids Li2CO3, Na2CO3, and K2CO3. These results are combined with calorimetric data to calculate the fusion curves for Li2CO3, Na2CO3, and K2CO3 to 5 kbar; the calculations are in excellent agreement with experimental determinations of the respective melting reactions.









Similar content being viewed by others
References
Araki N, Matsuura M, Makino A, Hirata T, Kato Y (1987) Measurements of thermophysical properties of molten salts (mixtures of alkaline carbonate salts). P1–4, In: 8th Japan Symposium on Thermophysical Properties
Barrett LR, Thomas AG (1959) Surface tension and density measurements on molten glasses in the CaO–Al2O3 system. Soc Glass Tech J 43:179T-190T
Birch F (1978) Finite strain isotherm and velocities for single-crystal and polycrystalline NaCl at high-pressures and 300-degree-K. J Geophys Res 83:1257–1268
Brooker RA, Hamilton DL (1990) Three-liquid immiscibility and the origin of carbonatites. Nature 346:459–462
Brooker RA, Kohn SC, Holloway JR, McMillan PF (2001a) Structural controls on the solubility of CO2 in silicate melts. Part I: bulk solubility data. Chem Geol 174:225–239
Brooker RA, Kohn SC, Holloway JR, McMillan PF (2001b) Structural controls on the solubility of CO2 in silicate melts. Part II: IR characteristics of carbonate groups in silicate glasses. Chem Geol 174:241–254
Brouns E, Visser JW (1964) An anomaly in the crystal structure of Na2CO3. Acta Crystallogr 17:614
Carmichael ISE, Turner FJ, Verhoogen J (1974) Igneous petrology. McGraw-Hill, New York, 739 pp
Church AA, Jones AP (1995) Silicate-carbonate immiscibility at Oldoinyo Lengai. J Petrol 36:869–889
Dingwell DB, Brearley M (1988) Melt densities in the CaO–FeO–Fe2O3–SiO2 system and the compositional dependence of the partial molar volume of ferric iron in silicate melts. Geochim Cosmochim Acta 52:2815–2825
Dobson DP, Jones AP, Rabe R, Toshimori S, Kurita K, Taniguchi T, Kondo T, Kato T, Shimomura O, Urakawa S (1996) In-situ measurement of viscosity and density of carbonate melts at high pressure. Earth Planet Sci Lett 143:207–215
Fei Y (1995) Thermal expansion. In: Ahren T (ed) Mineral physics and crystallography. AGU Reference Shelf 2, Washington, DC pp 29–44
Genge MJ, Price GD, Jones AP (1995) Molecular dynamics simulations of CaCO3 melts to mantle pressures and temperatures: implications for carbonatite magmas. Earth Planet Sci Lett 131:225–238
Janz GJ (1992) NIST properties of molten salts database. Version 2, Single salts and salt mixtures database, density, viscosity, electrical conductance, and surface tension. NIST Standard Reference Database 27
Janz GJ, Lorenz MR (1961) Molten carbonate electrolytes—physical properties, structure, and mechanism of electrical conductance. J Electrochem Soc 108:1052–1058
Klement W, Cohen LH (1975) Solid-solid and solid-liquid transitions in K2CO3, Na2CO3 and Li2CO3 : investigations to greater than ≥5 kbar by differential thermal-analysis—thermodynamics and structural correlations. Ber Bunsen Phys Chem 79:327–334
Kojima T, Yanagida M, Tanimoto K, Tamiya Y, Matsumoto H, Miyazaki Y (1999) The surface tension and the density of molten binary alkali carbonate systems. Electrochemistry 67:593–602
Koster van Groos AF, Wyllie PJ (1966) Liquid immiscibility in the system Na2O-Al2O3-SiO2-CO2 at pressures to 1 kilobar. Am J Sci 264:234–255
Kress VC, Carmichael ISE (1991) The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib Mineral Petrol 108:82–92
Lange RA (1997) A revised model for the density and thermal expansivity of K2O–Na2O–CaO–MgO–Al2O3–SiO2 liquids from 700 to 1,900 K: extension to crustal magmatic temperatures. Contrib Mineral Petrol 130:1–11
Lange (2003) The fusion curve of albite revisited and the compressibility of NaAlSi3O8 liquid with pressure. Am Mineral 88:109–120
Lange RA, Carmichael ISE (1987) Densities of Na2O–K2O–CaO–MgO–FeO–Fe2O3–TiO2–SiO2 liquids: new measurements and derived partial molar properties. Geochim Cosmochim Acta 51:2931–2946
Lange RA, Carmichael ISE (1990) Thermodynamic properties of silicate liquids with an emphasis on density, thermal expansion and compressibility. In: Nicholls J, Russell K (eds) Reviews in mineralogy, Mineralogical Society of America 24:25–64
Liu Q, Lange RA (2001) The partial molar volume and thermal expansivity of TiO2 in alkali silicate melts: systematic variation with Ti coordination. Geochim Cosmochim Acta 65:2379–2393
Ochs FA, Lange RA (1999) The density of hydrous magmatic liquids. Science 283:1314–1317
Pan V, Holloway JR, Hervig RL (1991) The pressure and temperature-dependence of carbon-dioxide solubility in tholeiitic basalt melts. Geochim Cosmochim Acta 55:1587–1595
Rigden SM, Ahrens TJ, Stolper EM (1989) High-pressure equation of state of molten anorthite and diopside. J Geophys Res 94:9508–9522
Rolin M, Recapet JM (1964) Contribution a l'étude des proprietes thermódynamiques des carbonates alcalins. III. Courbes d'enthalpies en fonction de la température et chaleurs de fusion de Na2CO3, Li2CO3, K2CO3 et de leur mélange eutectique ternaire. B Soc Chim Fr 10:2504–2510
Schneider SJ, Levin EM (1973) Polymorphism of K2CO3. J Am Ceram Soc 56:218–219
Smyth JR, McCormick TC (1995) Crystallographic data for minerals. In: Ahren T (ed) Mineral physics and crystallography. AGU Ref Shelf 2, Washington, DC, pp 29–44
Spedding PL (1970) Densities and molar volumes of molten alkali carbonate binary mixtures J Electrochem Soc 117:177–183
Stein DJ, Stebbins JF, Carmichael ISE (1986) Density of molten sodium aluminosilicates. J Am Ceram Soc 69:396–399
Stixrude L, Bukowinski MST (1990) Fundamental thermodynamic relations and silicate melting with implications for the constitution of D''. J Geophys Res 95:19311–19327
Stolper E, Holloway JR (1988) Experimental-determination of the solubility of carbon-dioxide in molten basalt at low-pressure. Earth Planet Sci Lett 87:397–408
Thibault Y, Holloway JR (1994) Solubility of CO2 in a Ca-rich leucitite—effects of pressure, temperature, and oxygen fugacity. Contrib Mineral Petrol 116:216–224
Treiman AH, Schedl A (1983) Properties of carbonatite magma and processes in carbonatite magma chambers. J Geol 91:437–447
Tuinstra F (1986) The thermal expansion of Na2CO3. Z Kristallogr 177:155–164
Vorob'ev GV, Pal'guev SF, Karpache SV (1961) Density and electrical conductivity of the fused alkali metal carbonates. II. Systems Na2CO3-Li2CO3 and K2CO3-Li2CO3. Trans Inst Elektrokhim 2:115–120
Ward AT, Janz GJ (1965) Molten carbonate electrolytes: electrical conductance, density and surface tension of binary and ternary mixtures. Electrochim Acta 10:849–857
Wolff JA (1994) Physical properties of carbonatite magmas inferred from molten salt data, and application to extraction patterns from carbonate-silicate magma chambers. Geol Mag 131:145–153
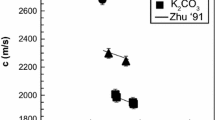
Zhu H, Saito T, Sato Y, Yamamura T, Shimakage K, Ejima T (1991) Ultrasonic velocity and absorption coefficient in molten alkali metal nitrates and carbonates. J Jpn Inst Metals 55:937–944
Acknowledgements
This research was supported by the National Science Foundation (EAR-9508133 and EAR-0087764). We wish to thank A. Kirfel and B. Barbier (Mineralogisch-Petrologisches Institut, Universität Bonn, Germany) for sharing their unpublished single-crystal X-ray diffraction data on the volume and thermal expansion of crystalline Li2CO3 at 1 bar. We especially thank Dr. D. Dobson for his e-mail correspondence, which helped us in our design of our density measurements of the CaCO3-bearing liquids under a stream of CO2. Helpful comments from D. Dobson, J. Wolff, and an anonymous reviewer significantly improved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: I Carmichael
Appendix
Appendix
Table 8 shows the density of the experimental liquids.
Rights and permissions
About this article
Cite this article
Liu, Q., Lange, R.A. New density measurements on carbonate liquids and the partial molar volume of the CaCO3 component. Contrib Mineral Petrol 146, 370–381 (2003). https://doi.org/10.1007/s00410-003-0505-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-003-0505-7