Abstract
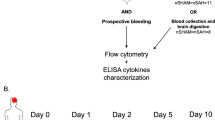
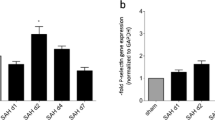
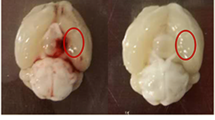
Inflammatory changes have been postulated to contribute to secondary brain injury after aneurysmal subarachnoid hemorrhage (SAH). In human specimens after SAH as well as in experimental SAH using mice, we show an intracerebral accumulation of inflammatory cells between days 4 and 28 after the bleeding. Using bone marrow chimeric mice allowing tracing of all peripherally derived immune cells, we confirm a truly CNS-intrinsic, microglial origin of these immune cells, exhibiting an inflammatory state, and rule out invasion of myeloid cells from the periphery into the brain. Furthermore, we detect secondary neuro-axonal injury throughout the time course of SAH. Since neuronal cell death and microglia accumulation follow a similar time course, we addressed whether the occurrence of activated microglia and neuro-axonal injury upon SAH are causally linked by depleting microglia in vivo. Given that the amount of neuronal cell death was significantly reduced after microglia depletion, we conclude that microglia accumulation inflicts secondary brain injury after SAH.







Similar content being viewed by others
References
Ajami B, Bennett JL, Krieger C et al (2011) Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat Neurosci 14:1142–1149. doi:10.1038/nn.2887
Aktas O, Smorodchenko A, Brocke S et al (2005) Neuronal damage in autoimmune neuroinflammation mediated by the death ligand TRAIL. Neuron 46:421–432. doi:10.1016/j.neuron.2005.03.018
Aktas O, Ullrich O, Infante-Duarte C et al (2007) Neuronal damage in brain inflammation. Arch Neurol 64:185–189. doi:10.1001/archneur.64.2.185
Aungst SL, Kabadi SV, Thompson SM et al (2014) Repeated mild traumatic brain injury causes chronic neuroinflammation, changes in hippocampal synaptic plasticity, and associated cognitive deficits. J Cereb Blood Flow Metab 34:1223–1232. doi:10.1038/jcbfm.2014.75
Bauer J, Huitinga I, Zhao W et al (1995) The role of macrophages, perivascular cells, and microglial cells in the pathogenesis of experimental autoimmune encephalomyelitis. Glia 15:437–446. doi:10.1002/glia.440150407
Bendel P, Koivisto T, Hänninen T et al (2006) Subarachnoid hemorrhage is followed by temporomesial volume loss: MRI volumetric study. Neurology 67:575–582. doi:10.1212/01.wnl.0000230221.95670.bf
Bendel P, Koivisto T, Niskanen E et al (2009) Brain atrophy and neuropsychological outcome after treatment of ruptured anterior cerebral artery aneurysms: a voxel-based morphometric study. Neuroradiology 51:711–722. doi:10.1007/s00234-009-0552-5
Berg Vom J, Prokop S, Miller KR et al (2012) Inhibition of IL-12/IL-23 signaling reduces Alzheimer’s disease-like pathology and cognitive decline. Nat Med 18:1812–1819. doi:10.1038/nm.2965
Bessis A, Béchade C, Bernard D, Roumier A (2007) Microglial control of neuronal death and synaptic properties. Glia 55:233–238. doi:10.1002/glia.20459
Bogie JFJ, Stinissen P, Hendriks JJA (2014) Macrophage subsets and microglia in multiple sclerosis. Acta Neuropathol 128:191–213. doi:10.1007/s00401-014-1310-2
Bosche B, Graf R, Ernestus R-I et al (2010) Recurrent spreading depolarizations after subarachnoid hemorrhage decreases oxygen availability in human cerebral cortex. Ann Neurol 67:607–617. doi:10.1002/ana.21943
Budohoski KP, Czosnyka M, Smielewski P et al (2013) Cerebral autoregulation after subarachnoid hemorrhage: comparison of three methods. J Cereb Blood Flow Metab 33:449–456. doi:10.1038/jcbfm.2012.189
Chalouhi N, Ali MS, Jabbour PM et al (2012) Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32:1659–1676. doi:10.1038/jcbfm.2012.84
Clark JF, Loftspring M, Wurster WL, Pyne-Geithman GJ (2008) Chemical and biochemical oxidations in spinal fluid after subarachnoid hemorrhage. Front Biosci 13:1806–1812
Clark SR, McMahon CJ, Gueorguieva I et al (2008) Interleukin-1 receptor antagonist penetrates human brain at experimentally therapeutic concentrations. J Cereb Blood Flow Metab 28:387–394. doi:10.1038/sj.jcbfm.9600537
Chou SH-Y, Feske SK, Atherton J et al (2012) Early elevation of serum tumor necrosis factor-α is associated with poor outcome in subarachnoid hemorrhage. J Investig Med 60:1054–1058
Dengler J, Schefold JC, Graetz D et al (2008) Point-of-care testing for interleukin-6 in cerebro spinal fluid (CSF) after subarachnoid haemorrhage. Med Sci Monit 14:BR265–8
Dhar R, Diringer MN (2008) The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care 8:404–412. doi:10.1007/s12028-008-9054-2
Dreier JP, Major S, Manning A et al (2009) Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain 132:1866–1881. doi:10.1093/brain/awp102
Dumont AS, Dumont RJ, Chow MM et al (2003) Cerebral vasospasm after subarachnoid hemorrhage: putative role of inflammation. Neurosurgery 53:123–133 (discussion 133–135)
Fabriek BO, Van Haastert ES, Galea I et al (2005) CD163-positive perivascular macrophages in the human CNS express molecules for antigen recognition and presentation. Glia 51:297–305. doi:10.1002/glia.20208
Goldmann T, Wieghofer P, Müller PF et al (2013) A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat Neurosci 16:1618–1626. doi:10.1038/nn.3531
Graetz D, Nagel A, Schlenk F et al (2010) High ICP as trigger of proinflammatory IL-6 cytokine activation in aneurysmal subarachnoid hemorrhage. Neurol Res 32:728–735. doi:10.1179/016164109X12464612122650
Grathwohl SA, Kälin RE, Bolmont T et al (2009) Formation and maintenance of Alzheimer’s disease beta-amyloid plaques in the absence of microglia. Nat Neurosci 12:1361–1363. doi:10.1038/nn.2432
Greenhalgh AD, Brough D, Robinson EM et al (2012) Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech 5:823–833. doi:10.1242/dmm.008557
Hanafy KA (2013) The role of microglia and the TLR4 pathway in neuronal apoptosis and vasospasm after subarachnoid hemorrhage. J Neuroinflammation 10:83. doi:10.1186/1742-2094-10-83
Hansen-Schwartz J, Vajkoczy P, Macdonald RL et al (2007) Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol Sci 28:252–256. doi:10.1016/j.tips.2007.04.002
Hasan DM, Chalouhi N, Jabbour P et al (2013) Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc 2:e000019. doi:10.1161/JAHA.112.000019
Hasegawa Y, Suzuki H, Sozen T et al (2011) Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl 110:43–48. doi:10.1007/978-3-7091-0353-1_8
Hashimoto T, Meng H, Young WL (2006) Intracranial aneurysms: links among inflammation, hemodynamics and vascular remodeling. Neurol Res 28:372–380. doi:10.1179/016164106X14973
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14:463–477. doi:10.1038/nri3705
Heppner FL, Greter M, Marino D et al (2005) Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med 11:146–152. doi:10.1038/nm1177
Hoffmann O, Priller J, Prozorovski T et al (2007) TRAIL limits excessive host immune responses in bacterial meningitis. J Clin Invest 117:2004–2013. doi:10.1172/JCI30356
Johansson CB, Youssef S, Koleckar K et al (2008) Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol 10:575–583. doi:10.1038/ncb1720
Kasius KM, Frijns CJM, Algra A, Rinkel GJE (2010) Association of platelet and leukocyte counts with delayed cerebral ischemia in aneurysmal subarachnoid hemorrhage. Cerebrovasc Dis 29:576–583. doi:10.1159/000306645
Kierdorf K, Erny D, Goldmann T et al (2013) Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci 16:273–280. doi:10.1038/nn.3318
Kozai TDY, Vazquez AL, Weaver CL et al (2012) In vivo two-photon microscopy reveals immediate microglial reaction to implantation of microelectrode through extension of processes. J Neural Eng 9:066001. doi:10.1088/1741-2560/9/6/066001
Krabbe G, Halle A, Matyash V et al (2013) Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 8:e60921. doi:10.1371/journal.pone.0060921
Leibinger M, Müller A, Gobrecht P et al (2013) Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis 4:e609. doi:10.1038/cddis.2013.126
Macdonald RL (2014) Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 10:44–58. doi:10.1038/nrneurol.2013.246
Macdonald RL, Higashida RT, Keller E et al (2010) Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit Care 13:416–424. doi:10.1007/s12028-010-9433-3
Macdonald RL, Kassell NF, Mayer S et al (2008) Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39:3015–3021. doi:10.1161/STROKEAHA.108.519942
Mashaly HA, Provencio JJ (2008) Inflammation as a link between brain injury and heart damage: the model of subarachnoid hemorrhage. Cleve Clin J Med 75(suppl 2):S26–S30
McMenamin PG, Wealthall RJ, Deverall M et al (2003) Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localisation by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res 313:259–269. doi:10.1007/s00441-003-0779-0
Michaud J-P, Rivest S (2015) Anti-inflammatory signaling in microglia exacerbates Alzheimer’s disease-related pathology. Neuron 85:450–452. doi:10.1016/j.neuron.2015.01.021
Müller A, Brandenburg S, Turkowski K et al (2014) Resident microglia, and not peripheral macrophages, are the main source of brain tumor mononuclear cells. Int J Cancer. doi:10.1002/ijc.29379
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28:399–414. doi:10.1179/016164106X115008
Park S, Yamaguchi M, Zhou C et al (2004) Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke 35:2412–2417. doi:10.1161/01.STR.0000141162.29864.e9
Parkhurst CN, Yang G, Ninan I et al (2013) Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155:1596–1609. doi:10.1016/j.cell.2013.11.030
Penkowa M, Molinero A, Carrasco J, Hidalgo J (2001) Interleukin-6 deficiency reduces the brain inflammatory response and increases oxidative stress and neurodegeneration after kainic acid-induced seizures. Neuroscience 102:805–818
Pluta RM, Hansen-Schwartz J, Dreier J et al (2009) Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res 31:151–158. doi:10.1179/174313209X393564
Prinz M, Priller J, Sisodia SS, Ransohoff RM (2011) Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat Neurosci 14:1227–1235. doi:10.1038/nn.2923
Provencio JJ (2013) Inflammation in subarachnoid hemorrhage and delayed deterioration associated with vasospasm: a review. Acta Neurochir Suppl 115:233–238. doi:10.1007/978-3-7091-1192-5_42
Provencio JJ, Altay T, Smithason S et al (2011) Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol 232:94–100. doi:10.1016/j.jneuroim.2010.10.016
Provencio JJ, Fu X, Siu A et al (2010) CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit Care 12:244–251. doi:10.1007/s12028-009-9308-7
Prunell GF, Mathiesen T, Diemer NH, Svendgaard N-A (2003) Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 52:165–175 (discussion 175–176)
Ransohoff RM, Cardona AE (2010) The myeloid cells of the central nervous system parenchyma. Nature 468:253–262. doi:10.1038/nature09615
Sakai H, Horinouchi H, Tomiyama K et al (2001) Hemoglobin-vesicles as oxygen carriers: influence on phagocytic activity and histopathological changes in reticuloendothelial system. Am J Pathol 159:1079–1088. doi:10.1016/S0002-9440(10)61783-X
Sarrafzadeh A, Schlenk F, Gericke C, Vajkoczy P (2010) Relevance of cerebral interleukin-6 after aneurysmal subarachnoid hemorrhage. Neurocrit Care 13:339–346. doi:10.1007/s12028-010-9432-4
Schneider UC, Schiffler J, Hakiy N et al (2012) Functional analysis of pro-inflammatory properties within the cerebrospinal fluid after subarachnoid hemorrhage in vivo and in vitro. J Neuroinflammation 9:28. doi:10.1186/1742-2094-9-28
Schubert GA, Thomé C (2008) Cerebral blood flow changes in acute subarachnoid hemorrhage. Front Biosci 13:1594–1603
Smithason S, Moore SK, Provencio JJ (2012) Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarachnoid hemorrhage through a myeloid cell-dependent mechanism. Neurocrit Care 16:327–334. doi:10.1007/s12028-011-9651-3
Smithason S, Moore SK, Provencio JJ (2013) Low-dose lipopolysaccharide injection prior to subarachnoid hemorrhage modulates Delayed Deterioration associated with vasospasm in subarachnoid hemorrhage. Acta Neurochir Suppl 115:253–258. doi:10.1007/978-3-7091-1192-5_45
Stein SC, Browne KD, Chen X-H et al (2006) Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery 59:781–787. doi:10.1227/01.NEU.0000227519.27569.45 (discussion 787–788)
Tan Y, Uchida K, Nakajima H et al (2013) Blockade of interleukin 6 signaling improves the survival rate of transplanted bone marrow stromal cells and increases locomotor function in mice with spinal cord injury. J Neuropathol Exp Neurol 72:980–993. doi:10.1097/NEN.0b013e3182a79de9
Tiebosch IACW, Dijkhuizen RM, Cobelens PM et al (2013) Effect of interferon-β on neuroinflammation, brain injury and neurological outcome after experimental subarachnoid hemorrhage. Neurocrit Care 18:96–105. doi:10.1007/s12028-012-9692-2
Titova E, Ostrowski RP, Zhang JH, Tang J (2009) Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res 31:568–581. doi:10.1179/174313209X382412
Tulamo R, Frösen J, Junnikkala S et al (2006) Complement activation associates with saccular cerebral artery aneurysm wall degeneration and rupture. Neurosurgery 59:1069–1076. doi:10.1227/01.NEU.0000245598.84698.26 (discussion 1076–1077)
Varvel NH, Grathwohl SA, Baumann F et al (2012) Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci USA 109:18150–18155. doi:10.1073/pnas.1210150109
Xie X, Wu X, Cui J et al (2013) Increase ICAM-1 and LFA-1 expression by cerebrospinal fluid of subarachnoid hemorrhage patients: involvement of TNF-α. Brain Res 1512:89–96. doi:10.1016/j.brainres.2013.03.041
You W-C, Wang C-X, Pan Y-X et al (2013) Activation of nuclear factor-κB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS ONE 8:e60290. doi:10.1371/journal.pone.0060290
Zhang B, Gaiteri C, Bodea L-G et al (2013) Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153:707–720. doi:10.1016/j.cell.2013.03.030
Zhou Y, Martin RD, Zhang JH (2011) Advances in experimental subarachnoid hemorrhage. Acta Neurochir Suppl 110:15–21. doi:10.1007/978-3-7091-0353-1_3
Zipp F, Aktas O (2006) The brain as a target of inflammation: common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci 29:518–527. doi:10.1016/j.tins.2006.07.006
Acknowledgments
This work was supported by the Deutsche Forschungsgemeinschaft (SFB TRR 43 and NeuroCure Exc 257 to FLH and PV, as well as HE 3130/6-1 to FLH) and the Federal Ministry of Education and Research (DLR/BMBF; Kompetenznetz Degenerative Demenzen to FLH). We thank Dr. Jana Glumm and Dr. Kelly Miller for kindly providing GFP-mice and CD11b-HSVTK mice, respectively. We thank Melina Nieminen-Kelhä and Irina Kremenetskaia for their constant technical support in the lab. We thank Adnan Ghori for advice in PCR issues.
Conflict of interest
The authors have declared that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schneider, U.C., Davids, AM., Brandenburg, S. et al. Microglia inflict delayed brain injury after subarachnoid hemorrhage. Acta Neuropathol 130, 215–231 (2015). https://doi.org/10.1007/s00401-015-1440-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-015-1440-1