Abstract
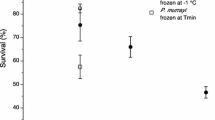
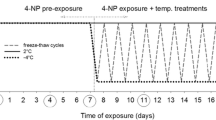
Panagrolaimus davidi is an Antarctic nematode with very high levels of cold tolerance. Its survival was compared with that of some other nematodes (P. rigidus, Rhabditophanes sp., Steinernema carpocapsae, Panagrellus redivivus and Ditylenchus dipsaci) in both unacclimated samples and those acclimated at 5°C. Levels of recrystallization inhibition in homogenates were also compared, using the splat-cooling assay. The survival of P. davidi after the freezing of samples was notably higher than that of the other species tested, suggesting that its survival ability is atypical compared to other nematodes. In general, acclimation improved survival. Levels of recrystallization inhibition were not associated with survival but such a relationship may exist for those species that are freezing tolerant.




Similar content being viewed by others
Abbreviations
- ATW:
-
Artificial tap water
- RMP:
-
Relative medium potency
- S 50 :
-
50% Survival temperature
- T min :
-
Minimum temperature
References
Andrassy I (1998) Nematodes in the sixth continent. J Nematode Morphol System 1:107–186
Behm CA (1997) The role of trehalose in the physiology of nematodes. Int J Parasitol 27:215–229
Brown IM, Gaugler R (1996) Cold tolerance of steinernematid and heterorhabditid nematodes. J Therm Biol 21:115–121
Brown IM, Gaugler R (1998) Survival of steinernematid nematodes exposed to freezing. J Therm Biol 23:75–80
Brown IM, Lovett BJ, Grewal PS, Gaugler R (2002) Latent infection: a low temperature survival strategy in steinernematid nematodes. J Therm Biol 27:531–539
Brown IM, Wharton DA, Millar RB (2005) The influence of temperature on the life history of the Antarctic nematode Panagrolaimus davidi. Nematology 6:883–890
Chen LB, Devries AL, Cheng CHC (1997) Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci USA 94:3811–3816
Curcic BPM, Sudhaus W, Dimitrijevic RN, Tomic VT, Curcic SB (2004) Phoresy of Rhabditophanes schneideri (Butschli) (Rhabditida: Alloionematidae) on pseudoscorpions (Arachnida: Pseudoscorpiones). Nematology 6:313–317
De Ley P, Mundo-Ocampo M (2004) Cultivation of nematodes. In: Chen ZX, Chen SY, Dickson DW (eds) Nematology: advances and perspectives, vol 1: nematode morphology, physiology and ecology. CABI Publishing, Wallingford, pp 541–619
Dorris M, Viney ME, Blaxter ML (2002) Molecular phylogenetic analysis of the genus Strongyloides and related nematodes. Int J Parasitol 32:1507–1517
Forge TA, MacGuidwin AE (1992) Effects of water potential and temperature on survival of the nematode Meloidogyne hapla in frozen soil. Can J Zool 70:1553–1560
Franks F, Mathias SF, Hatley RH (1990) Water, temperature and life. Philos Trans R Soc Lond B 326:517–533
Greenaway P (1970) Sodium regulation in the freshwater mollusc Limnaea stagnalis (L) (Gastropoda, Pulmonata). J Exp Biol 53:147–163
Holmstrup M, Westh P (1994) Dehydration of earthworm cocoons exposed to cold: a novel cold hardiness mechanism. J Comp Physiol B 164:312–315
Holmstrup M, Bayley M, Ramløv H (2002) Supercool or dehydrate? An experimental analysis of overwintering strategies in small permeable arctic invertebrates. Proc Natl Acad Sci USA 99:5716–5720
Hominick WM (2002) Biogeography. In: Gaugler R (ed) Entomopathogenic nematology. CRC, Boca Raton, pp 115–144
Hooper DJ (1986) Extraction of free-living stages from soil. In: Southey JF (ed) Laboratory methods for work with plant and soil nematodes. HMSO, London, pp 5–30
Knight CA, Hallett J, DeVries AL (1988) Solute effects on ice recrystallisation: an assessment technique. Cryobiology 25:55–60
Knight CA, Wen D, Laursen RA (1995) Nonequilibrium antifreeze peptides and the recrystallization of ice. Cryobiology 32:23–34
Lacey LA, Frutos R, Kaya HK, Vail P (2001) Insect pathogens as biological control agents: do they have a future? Biol Control 21:230–248
Lee RE (1991) Principles of insect low temperature tolerance. In: Lee RE, Denlinger DL (eds) Insects at low temperatures. Chapman & Hall, London, pp 17–46
Lee RE, McGrath JJ, Morason RT, Taddeo RM (1993) Survival of intracellular freezing, lipid coalescence and osmotic fragility in fat body cells of the freeze-tolerant gall fly Eurosta solidaginis. J Ins Physiol 39:445–450
Lees E (1953) An investigation into the method of dispersal of Pangrellus silusiae, with particular reference to its desiccation resistance. J Helminthol 27:95–103
Lewis EE, Shapiro-Ilan DI (2002) Host cadavers protect entomopathogenic nematodes during freezing. J Invertebr Pathol 81:25–32
Lewis EE, Gaugler R, Harrison R (1993) Response of cruiser and ambusher entomopathogenic nematodes (Steinernematidae) to host volatile cues. Can J Zool 71:765–769
Mabbett K, Wharton DA (1986) Cold tolerance and acclimation in the free-living nematode, Panagrellus redivivus. Revue Nématol 9:167–170
Moody EH, Lownsbery BF, Ahmed JH (1973) Culture of the root-lesion nematode Pratylenchus vulnus on carrot discs. J Nematol 5:225–226
Norusis MJ (1999) SPSS regression models 10.0. SPSS Inc, Chicago
Qiu LH, Bedding R (1999) Low temperature induced cryoprotectant synthesis by the infective juveniles of Steinernema carpocapsae: biological significance and mechanisms involved. Cryo Letters 20:393–404
Ramløv H, Wharton DA, Wilson PW (1996) Recrystallization in a freezing tolerant Antarctic nematode, Panagrolaimus davidi, and an alpine weta, Hemideina maori (Orthoptera, Stenopelmatidae). Cryobiology 33:607–613
Rosinski J, Langer G, Nagamoto CT, Banyard MC, Parungo FP (1976) Chemical composition of surfaces of natural ice-forming nuclei. J Atmos Res 10:201–210
Schmiege DC (1963) The feasibility of using a Neoaplectanid nematode for control of some forest insect pests. J Econ Entomol 56:427–431
Shannon AJ, Browne JA, Boyd J, Fitzpatrick DA, Burnell AM (2005) The anhydrobiotic potential and molecular phylogenetics of species and strains of Panagrolaimus (Nematoda, Panagrolaimidae). J Exp Biol 208:2433–2445
Stiernagle T (1999) Maintenance of C. elegans. In: Hope IA (ed) C. elegans: a practical approach. Oxford University Press, Oxford, pp 51–67
Storey KB, Storey JM (1988) Freeze tolerance in animals. Physiol Rev 68:27–84
Sturhan D, Brzeski MW (1991) Stem and bulb nematodes, Ditylenchus spp. In: Nickle WR (ed) Manual of agricultural nematology. Marcel Dekker, New York, pp 423–464
Subbotin SA, Madani M, Krall E, Sturhan D, Moens M (2005) Molecular diagnostics, taxonomy, and phylogeny of the stem nematode Ditylenchus dipsaci species complex based on the sequences of the internal transcribed spacer-rDNA. Phytopathol 95:1308–1315
Wharton DA (1995) Cold tolerance strategies in nematodes. Biol Rev 70:161–185
Wharton DA (1996) Water loss and morphological changes during desiccation of the anhydrobiotic nematode Ditylenchus dipsaci. J Exp Biol 199:1085–1093
Wharton DA (2002a) Life at the limits: organisms in extreme environments. Cambridge University Press, Cambridge
Wharton DA (2002b) Survival strategies. In: Lee DL (ed) The biology of nematodes. Taylor & Francis, London, pp 389–411
Wharton DA (2003) The environmental physiology of Antarctic terrestrial nematodes: a review. J Comp Physiol B 173:621–628
Wharton DA, Block W (1993) Freezing tolerance of some Antarctic nematodes. Func Ecol 7:578–584
Wharton DA, Brown IM (1991) Cold tolerance mechanisms of the Antarctic nematode Panagrolaimus davidi. J Exp Biol 155:629–641
Wharton DA, Ferns DJ (1995) Survival of intracellular freezing by the Antarctic nematode Panagrolaimus davidi. J Exp Biol 198:1381–1387
Wharton DA, Surrey MR (1994) Cold tolerance mechanisms of the infective larvae of the insect parasitic nematode, Heterorhabditis zealandica Poinar. Cryo Letters 25:749–752
Wharton DA, Young SR, Barrett J (1984) Cold tolerance in nematodes. J Comp Physiol B 154:73–77
Wharton DA, Judge KF, Worland MR (2000) Cold acclimation and cryoprotectants in a freeze-tolerant Antarctic nematode, Panagrolaimus davidi. J Comp Physiol B 170:321–327
Wharton DA, Goodall G, Marshall CJ (2003) Freezing survival and cryoprotective dehydration as cold tolerance mechanisms in the Antarctic nematode Panagrolaimus davidi. J Exp Biol 206:215–221
Wharton DA, Mutch JS, Wilson PW, Marshall CJ, Lim M (2004) A simple ice nucleation spectrometer. Cryo Letters 25:335–340
Wharton DA, Barrett J, Goodall G, Marshall CJ, Ramløv H (2005a) Ice-active proteins from the Antarctic nematode Panagrolaimus davidi. Cryobiology 51:198–207
Wharton DA, Downes MF, Goodall G, Marshall CJ (2005b) Freezing and cryoprotective dehydration in an Antarctic nematode (Panagrolaimus davidi) visualised using a freeze substitution technique. Cryobiology 50:21–28
Acknowledgments
We would like to thank Brian Niven for advice on probit analysis and the following for the supply of nematodes and/or assistance: D. dipsaci, Sharyn Taylor (SARDI, Adelaide) material for our initial infections; P. davidi, Antarctica NZ for supporting our Antarctic studies; S. carpocapsae, Tracey Nelson and Trevor Jackson (CASC, Lincoln); Rhabditophanes sp., Paul De Ley, Manuel Mundo-Ocampo and Jim Baldwin (UCLA Riverside) and for other species supplied but not used in this study; P. rigidus, Theresa Stiernagle (Caenorhabditis Genetics Center, funded by the NIH NCRR). DAW would like to thank Rick Lee Jr., Juanita Constible, Michael Elnitsky and Marcia Lee for their help during the part of this study conducted during his study leave at Rick Lee Jr’s laboratory. This study was made possible by a University of Otago Research Grant and by a Fullbright NZ Travel Award to DAW and complies with the laws regarding animal experimentation in New Zealand.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by I.D. Hume.
Rights and permissions
About this article
Cite this article
Smith, T., Wharton, D.A. & Marshall, C.J. Cold tolerance of an Antarctic nematode that survives intracellular freezing: comparisons with other nematode species. J Comp Physiol B 178, 93–100 (2008). https://doi.org/10.1007/s00360-007-0202-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-007-0202-3