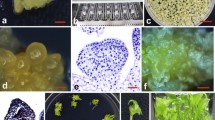
Abstract
A particle inflow gun was used to transfer the plasmid pAHC25 containing the bar gene conferring resistance to glufosinate and the gusA reporter gene, each driven by the maize ubiquitin promoter, to mature embryos of Pinus roxburghii (chir pine). High levels of transient expression were obtained when embryos were cultured for 6 days on 10 μM benzyl adenine-containing medium and then exposed to high osmoticum (0.5 M sucrose) before and after bombardment. Selection on medium containing Basta enabled recovery of stably transformed shoots, both from the epicotyl and from adventitious buds. The primary transformed shoots from the epicotyl were multiplied via axillary shoots. Transformation was confirmed by histochemical staining for β-glucuronidase (GUS) activity, by polymerase chain reaction (PCR) amplification of fragments of gusA and nos terminator, and by the resistance of needles to Basta.




Similar content being viewed by others
Abbreviations
- GUS :
-
β-glucuronidase
- X-Gluc :
-
5-bromo-4-choloro-3-indolyl-β-d-glucuronic acid
- BA :
-
benzylaminopurine
References
Aronen TS, Nikkanen TO, Häggman HM (2003) The production of transgenic Scots pine (Pinus sylvestris L.) via the application of transformed pollen in controlled crossings. Transgen Res 12:375–378
Bishop-Hurley SL, Zabkiewicz RJ, Grace L, Gardner RC, Wagner A, Walter C (2001) Conifer genetic engineering: transgenic Pinus radiata (D. Don) and Picea abies (Karst) plants are resistant to the herbicide Buster. Plant Cell Rep 20:235–243
Brukhin V, Clapham D, Elfstrand M, von Arnold S (2000) Basta tolerance as a selectable and screening marker for transgenic plants of Norway spruce. Plant Cell Rep 19:899–903
Charity JA, Holland L, Donaldson SS, Grace L, Walter C (2002) Agrobacterium-mediated transformation of Pinus radiata organogenic tissue using vacuum-infiltration. Plant Cell Tissue Org Cult 70:51–60
Christensen AH, Quail PH (1996) Ubiquitin promoter-based vectors for high-level expression of selectable and/or screenable marker genes in monocotyledonous plants. Transgen Res 5:213–218
Clapham D, Damel P, Elfstrand M, Koop H-U, Sabala I, von Arnold S (2000) Gene transfer by particle bombardment to embryogenic cultures of Picea abies and the production of transgenic plantlets. Scand J Forest Res 15:151–160
Doyle J, Doyle J (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Fernando DD, Owens JN, Misra S (2000) Transient gene expression in pine pollen tubes following particle bombardment. Plant Cell Rep 19:224–228
Finer JJ, Vain P, Jones MW, McMulley MD (1992) Development of the particle inflow gun for DNA delivery to plant cells. Plant Cell Rep 11:323–328
Gould JH, Zhou Y, Padmanabhan V, Magallanes-Cedeno ME, Newton RJ (2002) Transformation and regeneration of loblolly pine: shoot apex inoculation with Agrobacterium. Mol Breed 10:131–141
Grant JE, Cooper PA, Dale TM (2004) Transgenic Pinus radiata from Agrobacterium tumefactions-mediated transformation of cotyledons. Plant Cell Rep 22:894–902
Gupta PK, Durzan DJ (1985) Shoot multiplication from mature trees of Douglas-fir (Pseudotsuga menziesii) and sugar pine (Pinus lambertiana). Plant Cell Rep 4:177–179
Häggman HM, Aronen TS, Nikkanen TO (1997) Gene transfer by particle bombardment to Norway spruce and Scots pine pollen. Can J Forest Res 27:928–935
Hawkins S, Samaj J, Lauvergeat V, Boudet A, Grima-Pettenati J (1997) Cinnamyl alcohol dehydrogenase: identification of new sites of promoter activity in transgenic poplar. Plant Physiol 113:321–325
Jefferson RA (1987) Assaying chimeric plant genes: the GUS fusion system. Plant Mol Biol Rep 5:387–405
Jonard R (1986) Micrografting and its application to tree improvement. In: Bajaj YPS (ed) Biotechnology in agriculture and forestry, vol 1. Trees I. Springer, Berlin Heidelberg New York pp 31–48
Mathur G, von Arnold S, Nadgauda RS (2000) Somatic embryogenesis in Pinus roxburghii. Curr Sci 79:999–1004
Muriithi WT, Harry IS, Yeung EC, Thorpe TA (1993) Plantlet regeneration in chir pine (Pinus roxburghii Sarg.) – morphogenesis and histology. Forest Ecol Manage 57:141–160
Parasharami VA, Poonawala IS, Nadgauda RS (1999) Micropropagation of some Pinus species. Paper presented in international tree biotechnology meeting, Pune, India, November 17–19
Parasharami VA, Clapham D, Poonawala IS, von Arnold S, Nadgauda RS (2002) Transient gene expression in two pine species by microprojectile bombardment. Plant Cell Biotechnol Mol Biol 3:43–50
Parasharami VA, Poonawala IS, Nadgauda RS (2003) Bud break and plantlet regeneration in-vitro from mature trees of Pinus roxburghii Sarg. Curr Sci 84:203–208
Rey M, Gonzalez MV, Ordas RJ, Tavazza R, Ancora G (1996) Factors affecting transient gene expression in cultured radiata pine cotyledons following particle bombardment. Physiol Plant 96:630–636
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Stomp AM, Weissinger A, Sederoff RR (1991) Transient expression from microprojectile-mediated DNA transfer in Pinus taeda. Plant Cell Rep 10:187–190
Strauss SH, Brunner AM (2004) Tree biotechnology in the 21st century: transforming trees in the light of comparative genomics. In: Strauss SH, Bradshaw HD (eds) The bioengineered forests: challenges to science and society. Resources for the future. Washington, DC, pp 76–97
Tang W, Newton RJ (2003) Genetic transformation of conifers and its application in forest biotechnology. Plant Cell Rep 22:1–15
Tang W, Sederoff R, Whetten R (2001) Regeneration of transgenic loblolly pine (Pinus taeda L.) from zygotic embryos transformed with Agrobacterium tumefaciens. Planta 213:981–989
Tian L, Séguin A, Charest PJ (1997) Expression of the green fluorescent protein gene in conifer tissues. Plant Cell Rep 16:267–271
Tewari DN (1994) A monograph on chir pine (Pinus roxburghii Sarg.). International Book Distributors, Dehradun-248001, India, pp 1–311
Walter C, Smith DR, Connett MB, Grace L, White DWR (1994) A biolistic approach for the transfer and expression of a gusA reporter gene in embryonic cultures of Pinus radiata. Plant Cell Rep 14:69–74
Walter C, Grace LJ, Wagner A, White DWR, Walden AR, Donaldson SS, Hinton H, Gardner RC, Smith DR (1998) Stable transformation and regeneration of transgenic plants of Pinus radiata D. Don. Plant Cell Rep 17:460–468
Walter C, Charity J, Grace L, Höfig K, Möller R, Wagner A (2002) Gene technologies in Pinus radiata and Picea abies: tools for conifer biotechnology in the 21st century. Plant Cell Tissue Org Cult 70:3–12
Acknowledgments
The work was supported by Swedish International Development Agency (SIDA), Sweden, and by Department of Biotechnology (DBT), India
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.S. Petersen
Rights and permissions
About this article
Cite this article
Parasharami, V.A., Naik, V.B., von Arnold, S. et al. Stable transformation of mature zygotic embryos and regeneration of transgenic plants of chir pine (Pinus roxbughii Sarg.). Plant Cell Rep 24, 708–714 (2006). https://doi.org/10.1007/s00299-005-0019-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-005-0019-z