Abstract
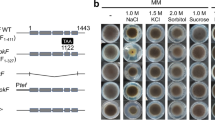
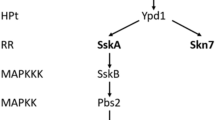
Ssk1- and Skn7-type response regulators are widely conserved in fungal His–Asp phosphorelay (two-component) signaling systems. SrrA, a Skn7-type RR of Aspergillus nidulans, is implicated not only in oxidative stress responses but also in osmotic adaptation, conidia production (asexual development), inhibition by fungicides, and cell wall stress resistance. Here, we characterized SrrA, focusing on the role of the conserved aspartate residue in the receiver domain, which is essential for phosphorelay function. We constructed strains carrying an SrrA protein in which aspartate residue D385 was replaced with either asparagine (N) or alanine (A). These mutants exhibited normal conidiation and partial oxidative stress resistance. In osmotic adaptation, mutants with substitution at SrrA D385 showed as much sensitivity as ΔsrrA strains, suggesting that SrrA plays a role in osmotic stress adaptation in a phosphorelay-dependent manner. The SrrA D385 substitution mutants showed significant resistance to fungicides and cell wall stresses. These results together led us to conclude that the conserved aspartate residue has a substantial impact on SrrA function, and that SrrA plays a role in several aspects of cellular function via His–Asp phosphorelay circuitry in Aspergillus nidulans.








Similar content being viewed by others
References
Adams TH, Wieser JK, Yu JH (1998) Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev 62:35–54
Appleby JL, Parkinson JS, Bourret RB (1996) Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell 86:845–848
Appleyard MVCL, McPheat WL, Stark MJ (2000) A novel ‘two-component’ protein containing histidine kinase and response regulator domains required for sporulation in Aspergillus nidulans. Curr Genet 37:364–372
Asano Y, Hagiwara D, Yamashino T, Mizuno T (2007) Characterization of the bZip-type transcription factor NapA with special reference to oxidative stress response in Aspergillus nidulans. Biosci Biotechnol Biochem 71:1800–1803
Ashby MK, Houmard J (2006) Cyanobacterial two-component proteins: structure, diversity, distribution, and evolution. Microbiol Mol Biol Rev 70:472–509
Azuma N, Kanamaru K, Matsushika A, Yamashino T, Mizuno T, Kato M, Kobayashi T (2007) In vitro analysis of His-Asp phosphorelays in Aspergillus nidulans: the first direct biochemical evidence for the existence of His-Asp phosphotransfer systems in filamentous fungi. Biosci Biotechnol Biochem 71:2493–2502
Bahn YS, Kojima K, Cox GM, Heitman J (2006) A unique fungal two-component system regulates stress responses, drug sensitivity, sexual development, and virulence of Cryptococcus neoformans. Mol Biol Cell 17:3122–3135
Banno S, Noguchi R, Yamashita K, Fukumori F, Kimura M, Yamaguchi I, Fujimura M (2007) Roles of putative His-to-Asp signaling modules, HPT-1 and RRG-2, on viability and sensitivity to osmotic and oxidative stresses in Neurospora crassa. Curr Genet 51:197–208
Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, Fischer R (2005) The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol 15:1833–1838
Brown JL, North S, Bussey H (1993) SKN7, a yeast multicopy suppressor of a mutation affecting cell wall beta-glucan assembly encodes a product with domains homologous to prokaryotic two-component regulators and to heat shock transcription factors. J Bacteriol 175:6908–6915
Brown JL, Bussey H, Stewart RC (1994) Yeast Skn7 functions in a eukaryotic two-component regulatory pathway. EMBO J 13:5186–5194
Catlett NL, Yoder OC, Turgeon BG (2003) Whole-genome analysis of two-component signal transduction genes in fungal pathogens. Eukaryot Cell 2:1151–1161
Chauhan N, Calderone R (2008) Two-component signal transduction proteins as potential drug targets in medically important fungi. Infect Immun 76:4795–4803
Coenjaerts FEJ, Hoepelman AIM, Scharringa J, Aarts M, Ellerbroek PM, Bevaart L, van Strijp JAG, Janbon G (2006) The Skn7 response regulator of Cryptococcus neoformans is involved in oxidative stress signaling and augments intracellular survival in endothelium. FEMS Yeast Res 6:652–661
Furukawa K, Katsuno Y, Urao T, Yabe T, Yamada-Okabe T, Yamada-Okabe H, Yamagata Y, Abe K, Nakajima T (2002) Isolation and functional analysis of a gene, tcsB, encoding a transmembrane hybrid-type histidine kinase from Aspergillus nidulans. Appl Environ Microbiol 68:5304–5310
Hagiwara D, Yamashino T, Mizuno T (2004) Genome-wide comparison of the His-to-Asp phosphorelay signaling components of three symbiotic genera of Rhizobia. DNA Res 11:57–65
Hagiwara D, Asano Y, Marui J, Furukawa K, Kanamaru K, Kato M, Abe K, Kobayashi T, Yamashino T, Mizuno T (2007a) The SskA and SrrA response regulators are implicated in oxidative stress responses of hyphal and asexual spores in the phosphorelay signaling network of Aspergillus nidulans. Biosci Biotechnol Biochem 71:1003–1014
Hagiwara D, Matsubayashi Y, Marui J, Furukawa K, Yamashino T, Kanamaru K, Kato M, Abe K, Kobayashi T, Mizuno T (2007b) Characterization of the NikA histidine kinase implicated in the phosphorelay signal transduction of Aspergillus nidulans, with special reference to fungicide responses. Biosci Biotechnol Biochem 71:844–847
Hagiwara D, Kondo A, Fujioka T, Abe K (2008) Functional analysis of C2H2 zinc finger transcription factor CrzA involved in calcium signaling in Aspergillus nidulans. Curr Genet 54:325–338
He XJ, Fassler JS (2005) Identification of novel Yap1 and Skn7 binding sites involved in the oxidative stress response of Saccharomyces cerevisiae. Mol Microbiol 58:1454–1467
He XJ, Mulford KE, Fassler JS (2009) Oxidative stress function of the Saccharomyces cerevisiae Skn7 receiver domain. Eukaryot Cell 8:768–778
Hoch JA, Silhavy TJ (1995) Two-component signal transduction. ASM Press, Washington DC, pp 1–473
Ishida K, Niwa Y, Yamashino T, Mizuno T (2009) A genome-wide compilation of the two-component systems in Lotus japonicus. DNA Res 16:237–247
Izumitsu K, Yoshimi A, Tanaka C (2007) Two-component response regulators Ssk1p and Skn7p additively regulate high-osmolarity adaptation and fungicide sensitivity in Cochliobolus heterostrophus. Eukaryot Cell 6:171–181
Krems B, Charizanis C, Entian KD (1996) The response regulator-like protein Pos9/Skn7 of Saccharomyces cerevisiae is involved in oxidative stress resistance. Curr Genet 29:327–334
Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP (2007) Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Gent Biol 44:682–690
Lee J, Gordon C, Lagniel G, Spector D, Garin J, Labarre J, Toledano MB (1999) Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 274:16040–16046
Li S, Ault A, Malone CL, Raitt D, Dean S, Johnston LH, Deschenes RJ, Fassler JS (1998) The yeast histidine protein kinase, Sln1p, mediates phosphotransfer to two response regulators, Ssk1p and Skn7p. EMBO J 17:6952–6962
Li S, Dean S, Li Z, Horecka J, Deschenes RJ, Fassler JS (2002) The eukaryotic two-component histidine kinase Sln1p regulates OCH1 via the transcription factor Skn7p. Mol Biol Cell 13:412–424
Maeda T, Wuegler-Murphy SM, Saito H (1994) A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature 369:242–245
Mizuno T (1998) His-Asp phosphotransfer signal transduction. J Biochem (Tokyo) 123:555–563
Mizuno T (2005) Two-component phosphorelay signal transduction systems in plants: from hormone response to circadian rhythms. Biosci Biotechnol Biochem 69:2263–2276
Morgan BA, Banks GR, Toone WM, Raitt D, Kuge S, Johnston LH (1997) The Skn7 response regulator controls gene expression in the oxidative stress response of the budding yeast Saccharomyces cerevisiae. EMBO J 16:1035–1044
Motoyama T, Ochiai N, Morita M, Iida Y, Usami R, Kudo T (2008) Involvement of putative response regulator genes of the rice blast fungus Magnaporthe oryzae in osmotic stress response, fungicide action, and pathogenicity. Curr Genet 54:185–195
Nakamichi N, Yamada H, Aiba H, Aoyama K, Ohmiya R, Mizuno T (2003) Characterization of the Prr1 response regulator with special response to sexual development in Schizosaccharomyces pombe. Biosci Biotechnol Biochem 67:547–555
Narang SS, Malone CL, Deschenes RJ, Fassler JS (2008) Modulation of yeast Sln1 kinase activity by the Ccw12 cell wall protein. J Biol Chem 283:1962–1973
Navarro RE, Aguirre J (1998) Posttranscriptional control mediates cell type-specific localization of catalase A during Aspergillus nidulans development. J Bacteriol 180:5733–5738
Ohmiya R, Kato C, Yamada H, Aiba H, Mizuno T (1999) A fission yeast gene (prr1 +) that encodes a response regulator implicated in oxidative stress response. J Biochem 125:1061–1066
Ohmiya R, Yamada H, Kato C, Aiba H, Mizuno T (2000) The Prr1 response regulator is essential for transcription of ste11 + and for sexual development in fission yeast. Mol Gen Genet 264:441–451
Pareek A, Singh A, Kumar M, Kushwaha HR, Lynn AM, Singla-Pareek SL (2006) Whole-genome analysis of Oryza sativa reveals similar architecture of two-component signaling machinery with Arabidopsis. Plant Physiol 142:380–397
Posas F, Saito H (1998) Activation of the yeast SSK2 MAP kinase kinase kinase by the SSK1 two-component response regulator. EMBO J 17:1385–1394
Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H (1996) Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 “two-component” osmosensor. Cell 86:865–875
Raitt DC, Johnson AL, Erkine AM, Makino K, Morgan B, Gross DS, Johnston LH (2000) The Skn7 response regulator of Saccharomyces cerevisiae interacts with Hsf1 in vivo and is required for the induction of heat shock genes by oxidative stress. Mol Biol Cell 11:2235–2247
Singh P, Chauhan N, Ghosh A, Dixon F, Calderone R (2004) SKN7 of Candida albicans: mutant construction and phenotype analysis. Infect Immun 72:2390–2394
Thön M, Al Abdallah Q, Hortschansky P, Scharf DH, Eisendle M, Haas H, Brakhage AA (2010) The CCAAT-binding complex coordinates the oxidative stress response in eukaryotes. Nucleic Acids Res 38:1098–1113
Vargas-Perez I, Sanchez O, Kawasaki L, Georgellis D, Aguirre J (2007) Response regulators SrrA and SskA are central components of a phosphorelay system involved in stress signal transduction and asexual sporulation in Aspergillus nidulans. Eukaryot Cell 6:1570–1583
Wormley FL Jr, Heinrich G, Miller JL, Perfect JR, Cox GM (2005) Identification and characterization of an SKN7 homologue in Cryptococcus neoformans. Infect Immun 73:5022–5030
Zhang W, Shi L (2005) Distribution and evolution of multiple-step phosphorelay in prokaryotes: lateral domain recruitment involved in the formation of hybrid-type histidine kinases. Microbiol 151:2159–2173
Acknowledgments
D. Hagiwara was supported by a research fellowship from the Japan Society for the Promotion of Science. K. Abe was supported in part by grants from the Bio-oriented Technology Research Advancement Institution (BRAIN).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by A. Brakhage.
An erratum to this article can be found at http://dx.doi.org/10.1007/s00294-011-0337-3
Electronic supplementary material
Below is the link to the electronic supplementary material.
294_2010_330_MOESM1_ESM.ppt
Fig. S1 Sequence analyses of the HSF and receiver domains of SrrA. a Alignment of heat-shock-factor domains of SrrA. The amino acid sequence of HSF of SrrA was aligned with those of other fungi by the Clustal X program. The numbers shown at the ends of each sequence indicate positions from the initiation peptide. b Phylogenetic tree of receiver domain of the fungal Skn7-type RRs. The phylogenetic tree was built by using Tree View software based on sequence alignment of the receiver domains of SrrA orthologs (Table 1).(PPT 115 kb)
Rights and permissions
About this article
Cite this article
Hagiwara, D., Mizuno, T. & Abe, K. Characterization of the conserved phosphorylation site in the Aspergillus nidulans response regulator SrrA. Curr Genet 57, 103–114 (2011). https://doi.org/10.1007/s00294-010-0330-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00294-010-0330-2