Abstract
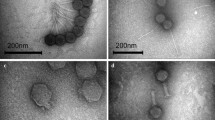
Characterization of bacteriophages to be used prophylactically or therapeutically is mandatory, as use of uncharacterized bacteriophages is considered as one of the major reasons of failure of phage therapy in preantibiotic era. In the present study, one lytic bacteriophage, KPO1K2, specific for Klebsiella pneumoniae B5055, with broad host range was selected for characterization. As shown by TEM, morphologically KPO1K2 possessed icosahedral head with pentagonal nature with apex to apex head diameter of about 39 nm. Presence of short noncontractile tail (10 nm) suggested its inclusion into family Podoviridae with a designation of T7-like lytic bacteriophage. The phage growth cycle with a latent period of 15 min and a burst size of approximately 140 plaque forming units per infected cell as well as a genome of 42 kbps and structural protein pattern of this bacteriophage further confirmed its T7-like characteristics. Phage was stable over a wide pH range of 4–11 and demonstrated maximum activity at 37°C. After injection into mice, at 6 h, a high phage titer was seen in blood as well as in kidney and urinary bladder, though titers in kidney and urinary bladder were higher as compared to blood. Phage got cleared completely in 36 h from blood while from kidneys and urinary bladder its clearance was delayed. We propose the use of this characterized phage, KPO1K2, as a prophylactic/therapeutic agent especially for the treatment of catheter associated UTI caused by Klebsiella pneumoniae.







Similar content being viewed by others
References
Ackermann H-W (2005) Bacteriophage classification. In: Kutter E, Sulakvelidze A (eds) Bacteriophages biology, applications. CRC Press, Boca Raton
Ackermann H-W, Dubow MS, Jarvis AW et al (1992) The species concept and its application to tailed phages. Arch Viol 124:69–82
Adams MH (1959) Bacteriophages. Interscience, New York
Ashelford KE, Norris SJ, Fry JC et al (2000) Seasonal population dynamics and interactions of competing bacteriophages and their host in the rhizosphere. Appl Environ Microbiol 66:4193–4199
Ausubel F, Brent R, Kingston RE et al (2001) Current protocols in molecular biology. Wiley and Sons, NY, New York
Bedi MS, Verma V, Chhibber S (2009) Amoxicillin and specific bacteriophage can be used together for the eradication of biofilm of Klebsiella pneumoniae B5055. World J Microbiol Biotechnol (Accepted for publication). doi: 10.1007/s11274-009-9991-8
Bruttin A, Brussow H (2005) Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob Agents Chemother 49:2874–2878
Casjens SR (2008) Diversity among the tailed-bacteriophages that infect the Enterobacteriaceae. Res Microbiol 159(2008):340–348
Cerveny KE, DePaola A, Duckworth DH, Gulig PA (2002) Phage therapy of local and systemic disease caused by Vibrio vulnificus in iron-dextran-treated mice. Infect Immun 70(11):6251–6262
Chhibber S, Kaur S, Kumari S (2008) Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J Med Microbiol 57:1508–1513
Comeau AM, Tétart F, Trojet SN, Pre`re M-F, Krisch HM (2007) Phage-Antibiotic Synergy (PAS): b-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2(8):e799. doi:10.1371/journal.pone.0000799
Danese PN (2002) Antibiofilm approaches: review prevention of catheter colonization. Chem Biol 9:873–880
Geyer H, Himmelspach K, Kwiatkowski B et al (1983) Degradation of bacterial surface carbohydrates by virus-associated enzymes. Pure Appl Chem 55:637–653
Gilbert P, Das J, Foley I (1997) Biofilm susceptibility to antimicrobials. Adv Dent Res 11(1):160–167
Goodridge L, Gallaccio A, Griffiths MW (2003) Morphological, host range, and genetic characterization of two coliphages. Appl Environ Microbiol 69(9):5364–5371
Gorski A, Weber-Dąbrowska B (2005) The potential role of endogenous bacteriophages in controlling invading pathogens. Cell Mol Life Sci 62:511–519
Hendrix RW (2002) Bacteriophages: evolution of the majority. Theor Popul Biol 61:471–480
Holloway BW, Krishnapillai V, Morgan AF (1970) Chromosomal genetics of Pseudomonas. Microbiol Rev 43(1):73–102
Johnson JR, Kuskowski MA, Wilt TJ (2006) Systematic review: antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Ann Intern Med 144(2):117–126
Kumari S, Harjai K, Chhibber S (2008) Efficacy of bacteriophage treatment in murine burn wound infection induced by Klebsiella pneumoniae. J Microbiol Biotechnol (published online December). doi:10.4014/jmb.0808.493
Kutter E, Sulakvelidze A (2005) Bacteriophages biology and applications. CRC Press, Boca Raton
Lavigne R, Burkal’tseva MV, Robben J et al (2003) The genome of bacteriophage phi KMV, a T7-like virus infecting Pseudomonas aeruginosa. Virology 312:49–59
Maniatis T, Sambrook J, Fritsch EF (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Miedzybrodzki R, Switala-Jelen K, Fortuna W et al (2008) Bacteriophage inhibition of reactive oxygen species generation by endotoxin-stimulated polymorphonuclear leukocytes. Virus Res 131:233–242
Pajunen M, Kiljunen S, Skurnik M et al (2000) Bacteriophage phi YeO3-12, specific for Yersinia enterocolitica serotype O:3, is related to coliphages T3 and T7. J Bacteriol 182:5114–5120
Scholl D, Rogers S, Adhya S, Merril CR (2001) Bacteriophage K1-5 encodes two different tail fiber proteins, allowing it to infect and replicate on both K1 and K5 strains of Escherichia coli. J Virol 75(6):2509–2515
Sillankorva S, Neubauer P, Azeredo J (2008) Isolation and characterization of a T7-like lytic phage for Pseudomonas fluorescens. BMC Biotechnol 8:59–70
Sutherland IW (2001) Biofilm polysaccharide: a strong and sticky framework. Microbiology 147:3–9
Suttle CA (2007) Marine viruses—major players in the global ecosystem. Nat Rev Microbiol 5:801–812
Vinodkumar CS, Neelagund YF, Kalsurmath S (2005) Bacteriophage in the treatment of experimental septicaemia mice from a clinical isolate of multidrug resistant Klebsiella pneumoniae. J Commun Dis 37:18–29
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182(10):2675–2679
Weld R (2000) Bacteriophage on the menu. Pharm Sci Technol Today 3(12):404–405
Yamamoto KR, Alberts BM, Benzinger R et al (1970) Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology 40:734–744
Acknowledgment
This study was supported by the Indian Council of Medical Research (ICMR). We also thank Dr. Rupinder Tiwari for the facilities used in his laboratory pertaining to restriction digestion experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verma, V., Harjai, K. & Chhibber, S. Characterization of a T7-Like Lytic Bacteriophage of Klebsiella pneumoniae B5055: A Potential Therapeutic Agent. Curr Microbiol 59, 274–281 (2009). https://doi.org/10.1007/s00284-009-9430-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-009-9430-y