Abstract
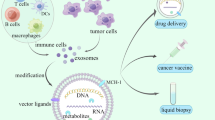
Glioblastomas are primary intracranial tumors for which there is no cure. Patients receiving standard of care, chemotherapy and irradiation, survive approximately 15 months prompting studies of alternative therapies including vaccination. In a pilot study, a vaccine consisting of Lucite diffusion chambers containing irradiated autologous tumor cells pre-treated with an antisense oligodeoxynucleotide (AS-ODN) directed against the insulin-like growth factor type 1 receptor was found to elicit positive clinical responses in 8/12 patients when implanted in the rectus sheath for 24 h. Our preliminary observations supported an immune response, and we have since reopened a second Phase 1 trial to assess this possibility among other exploratory objectives. The current study makes use of a murine glioma model and samples from glioblastoma patients in this second Phase 1 trial to investigate this novel therapeutic intervention more thoroughly. Implantation of the chamber-based vaccine protected mice from tumor challenge, and we posit this occurred through the release of immunostimulatory AS-ODN and antigen-bearing exosomes. Exosomes secreted by glioblastoma cultures are immunogenic, eliciting and binding antibodies present in the sera of immunized mice. Similarly, exosomes released by human glioblastoma cells bear antigens recognized by the sera of 6/12 patients with recurrent glioblastomas. These results suggest that the release of AS-ODN together with selective release of exosomes from glioblastoma cells implanted in chambers may drive the therapeutic effect seen in the pilot vaccine trial.






Similar content being viewed by others
Abbreviations
- AS-ODN:
-
Antisense oligodeoxynucleotide
- B-FGF:
-
Basic fibroblast growth factor
- DiO:
-
3,3′-dioctadecyloxacarbocyanine perchlorate
- DLN:
-
Draining lymph nodes
- EGF:
-
Epidermal growth factor
- FBS:
-
Fetal bovine serum
- GBM:
-
Glioblastoma
- GM-CSF:
-
Granulocyte macrophage colony stimulating factor
- IGF-1R:
-
Insulin-like growth factor type 1 receptor PBS
- IL:
-
Interleukin
- MFI:
-
Median fluorescence intensity
- mDC:
-
Myeloid dendritic cell
- PBMC:
-
Peripheral blood mononuclear cells
- PBS:
-
Phosphate buffered saline
- pDC:
-
Plasmacytoid dendritic cell
- Th2:
-
T helper type 2
References
Andrews DW, Resnicoff M, Flanders AE et al (2001) Results of a pilot study involving the use of an antisense oligodeoxynucleotide directed against the insulin-like growth factor type I receptor in malignant astrocytomas. J Clin Oncol 19:2189–2200
Baserga R, Reiss K, Alder H, Pietrzkowski Z, Surmacz E (1992) Inhibition of cell cycle progression by antisense oligodeoxynucleotides. Ann N Y Acad Sci 660:64–69
Hernandez-Sanchez C, Blakesley V, Kalebic T, Helman L, LeRoith D (1995) The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem 270:29176–29181
Resnicoff M, Abraham D, Yutanawiboonchai W et al (1995) The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res 55:2463–2469
Bauer S, Kirschning CJ, Hacker H et al (2001) Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98:9237–9242
Iho S, Yamamoto T, Takahashi T, Yamamoto S (1999) Oligodeoxynucleotides containing palindrome sequences with internal 5′-CpG-3′ act directly on human NK and activated T cells to induce IFN-gamma production in vitro. J Immunol 163:3642–3652
Kobayashi N, Hong C, Klinman DM, Shirota H (2013) Oligodeoxynucleotides expressing polyguanosine motifs promote antitumor activity through the upregulation of IL-2. J Immunol 190:1882–1889
Krieg AM, Yi AK, Matson S et al (1995) CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 374:546–549
Pasare C, Medzhitov R (2003) Toll pathway-dependent blockade of CD4+ CD25+ T cell-mediated suppression by dendritic cells. Science 299:1033–1036
Stein CA, Subasinghe C, Shinozuka K, Cohen JS (1988) Physicochemical properties of phosphorothioate oligodeoxynucleotides. Nucleic Acids Res 16:3209–3221
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420
Théry C, Boussac M, Véron P et al (2001) Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 166:7309–7318
Raposo G, Nijman HW, Stoorvogel W et al (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Wolfers J, Lozier A, Raposo G et al (2001) Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat Med 7:297–303
Clayton A, Court J, Navabi H et al (2001) Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J Immunol Methods 247:163–174
Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C (2005) Exosomal-like vesicles are present in human blood plasma. Int Immunol 17:879–887
Graner MW, Alzate O, Dechkovskaia AM et al (2009) Proteomic and immunologic analyses of brain tumor exosomes. FASEB J 23:1541–1557
Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G (2002) The biogenesis and functions of exosomes. Traffic 3:321–330
Théry C, Amigorena S, Raposo G, Clayton A (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. Chapter 3: Unit 3.22. doi: 10.1002/0471143030.cb0322s30
Faure J, Lachenal G, Court M et al (2006) Exosomes are released by cultured cortical neurones. Mol Cell Neurosci 31:642–648
Kramer-Albers EM, Bretz N, Tenzer S, Winterstein C (2007) Oligodendrocytes secrete exosomes containing major myelin and stress-protective proteins: trophic support for axons? Proteomics Clin Appl 1:1446–1461
Potolicchio I, Carven GJ, Xu X et al (2005) Proteomic analysis of microglia-derived exosomes: metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J Immunol 175:2237–2243
Zitvogel L, Regnault A, Lozier A et al (1998) Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat Med 4:594–600
Février B, Raposo G (2004) Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol 16:415–421
Al-Nedawi K, Meehan B, Micallef J et al (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10:619–624
Skog J, Wurdinger T, van Rijn S et al (2008) Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol 10:1470–1476
Wieckowski E, Whiteside TL (2006) Human tumor-derived vs dendritic cell-derived exosomes have distinct biologic roles and molecular profiles. Immunol Res 36:247–254
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Andre F, Schartz NE, Movassagh M et al (2002) Malignant effusions and immunogenic tumour-derived exosomes. Lancet 360:295–305
Chaput N, Schartz NE, André F et al (2004) Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J Immunol 172:2137–2146
Creagh EM, O’Neill LA (2006) TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends Immunol 27:352–357
Mignot G, Roux S, Thery C, Ségura E, Zitvogel L (2006) Prospects for exosomes in immunotherapy of cancer. J Cell Mol Med 10:376–388
Morelli AE (2006) The immune regulatory effect of apoptotic cells and exosomes on dendritic cells: its impact on transplantation. Am J Transplant 6:254–261
Peche H, Renaudin K, Beriou G, Merieau E, Amigorena S, Cuturi MC (2006) Induction of tolerance by exosomes and short-term immunosuppression in a fully MHC-mismatched rat cardiac allograft model. Am J Transpl 6:1541–1550
Tang J, Flomenberg P, Harshyne L, Kenyon L, Andrews DW (2005) Glioblastoma patients exhibit circulating tumor-specific CD8+ T cells. Clin Cancer Res 11:5292–5299
Abraham D, Rotman HL, Haberstroh HF et al (1995) Strongyloides stercoralis: protective immunity to third-stage larvae inBALB/cByJ mice. Exp Parasitol 80:297–307
Tavernier J, Tuypens T, Verhee A et al (1995) Identification of receptor-binding domains on human interleukin 5 and design of an interleukin 5-derived receptor antagonist. Proc Natl Acad Sci USA 92:5194–5198
Lee GR, Fields PE, Griffin TJ, Flavell RA (2003) Regulation of the Th2 cytokine locus by a locus control region. Immunity 19:145–153
Rolink AG, Thalmann P, Kikuchi Y, Erdei A (1990) Characterization of the interleukin 5-reactive splenic B cell population. Eur J Immunol 20:1949–1956
McHeyzer-Williams MG (1989) Combinations of interleukins 2, 4 and 5 regulate the secretion of murine immunoglobulin isotypes. Eur J Immunol 19:2025–2030
Valenti R, Huber V, Iero M, Filipazzi P, Parmiani G, Rivoltini L (2007) Tumor-released microvesicles as vehicles of immunosuppression. Cancer Res 67:2912–2915
Murphy KA, Erickson JR, Johnson CS et al (2014) CD8+ T cell-independent tumor regression induced by Fc-OX40L and therapeutic vaccination in a mouse model of glioma. J Immunol 192:224–233
Acknowledgments
Financial Support: The Albert Stevens Foundation (David W. Andrews).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Harshyne, L.A., Hooper, K.M., Andrews, E.G. et al. Glioblastoma exosomes and IGF-1R/AS-ODN are immunogenic stimuli in a translational research immunotherapy paradigm. Cancer Immunol Immunother 64, 299–309 (2015). https://doi.org/10.1007/s00262-014-1622-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-014-1622-z