Abstract
Defects in human leukocyte antigen class I antigen processing machinery (APM) component expression can have a negative impact on the clinical course of tumors and the response to T cell-based immunotherapy. Since brain metastases of breast cancer are of increasing clinical significance, the APM component expression levels and CD8+ T cell infiltration patterns were analyzed in primary breast and metastatic brain lesions of breast cancer by immunohistochemistry. Comparison of unpaired 50 primary and 33 brain metastases showed lower expression of β2-microglobulin, transporter associated with antigen processing (TAP) 1, TAP2 and calnexin in the brain lesions. Although no significant differences were found in APM component scores between primary breast and brain lesions in 15 paired cases, primary breast lesions of which patients eventually developed brain metastases showed lower levels of β2-microglobulin, TAP1 and calnexin compared with breast lesions without known brain metastases. The extent of CD8+ T cell infiltration was significantly higher in the lesions without metastasis compared with the ones with brain metastases, and was positively associated with the expression of TAP1 and calnexin. Furthermore, mouse tumor cells stably transfected with silencing hairpin (sh)RNA for TAP1 demonstrated a decreased susceptibility to cytotoxic T lymphocytes in vitro and enhanced spontaneous brain metastasis in vivo. These data support the functional significance of TAP1 expression in tumor cells. Taken together, our data suggest that patients with low or defective TAP1 or calnexin in primary breast cancers may be at higher risks for developing brain metastasis due to the defects in T cell-based immunosurveillance.





Similar content being viewed by others
References
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE (2004) Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the metropolitan detroit cancer surveillance system. J Clin Oncol 22(14):2865–2872. doi:10.1200/jco.2004.12.149
Patel RR, Mehta MP (2007) Targeted therapy for brain metastases: improving the therapeutic ratio. Clin Cancer Res 13(6):1675–1683. doi:10.1158/1078-0432.ccr-06-2489
Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS (2005) Breast cancer metastasis to the central nervous system. Am J Pathol 167(4):913–920
Lin NU, Bellon JR, Winer EP (2004) CNS metastases in breast cancer. J Clin Oncol 22(17):3608–3617. doi:10.1200/jco.2004.01.175
Stemmler H-J, Heinemann V (2008) Central nervous system metastases in HER-2-overexpressing metastatic breast cancer: a treatment challenge. Oncologist 13(7):739–750. doi:10.1634/theoncologist.2008-0052
Kennecke H, Yerushalmi R, Woods R, Cheang MCU, Voduc D, Speers CH, Nielsen TO, Gelmon K (2010) Metastatic behavior of breast cancer subtypes. J Clin Oncol 28(20):3271–3277. doi:10.1200/jco.2009.25.9820
Martin JJ, Kondziolka D (2005) Indications for resection and radiosurgery for brain metastases. Curr Opin Oncol 17(6):584–587
Kondziolka D, Kano H, Harrison GL, Yang H-C, Liew DN, Niranjan A, Brufsky AM, Flickinger JC, Lunsford LD (2010) Stereotactic radiosurgery as primary and salvage treatment for brain metastases from breast cancer. J Neurosurg. doi:10.3171/2010.8.JNS10461
Okada H, Kohanbash G, Zhu X, Kastenhuber ER, Hoji A, Ueda R, Fujita M (2009) Immunotherapeutic approaches for glioma. Crit Rev Immunol 29(1):1–42
Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, Mintz AH, Engh JA, Bartlett DL, Brown CK, Zeh H, Holtzman MP, Reinhart TA, Whiteside TL, Butterfield LH, Hamilton RL, Potter DM, Pollack IF, Salazar AM, Lieberman FS (2011) Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with α-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29(3):330–336. doi:10.1200/jco.2010.30.7744
Campoli M, Ferrone S (2008) HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene 27(45):5869–5885
Cabrera T, Maleno I, Collado A, Lopez Nevot MA, Tait BD, Garrido F (2007) Analysis of HLA class I alterations in tumors: choosing a strategy based on known patterns of underlying molecular mechanisms. Tissue Antigens 69:264–268. doi:10.1111/j.1399-0039.2006.00777.x
Cathro H, Smolkin M, Theodorescu D, Jo V, Ferrone S, Frierson H (2010) Relationship between HLA class I antigen processing machinery component expression and the clinicopathologic characteristics of bladder carcinomas. Cancer Immunol Immunother 59(3):465–472. doi:10.1007/s00262-009-0765-9
Raffaghello L, Nozza P, Morandi F, Camoriano M, Wang X, Garre ML, Cama A, Basso G, Ferrone S, Gambini C, Pistoia V (2007) Expression and functional analysis of human leukocyte antigen class I antigen-processing machinery in medulloblastoma. Cancer Res 67(11):5471–5478. doi:10.1158/0008-5472.can-06-4735
Vitale M, Rezzani R, Rodella L, Zauli G, Grigolato P, Cadei M, Hicklin DJ, Ferrone S (1998) HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res 58(4):737–742
Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP (1998) gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med 188(2):277–286. doi:10.1084/jem.188.2.277
Stam N, Spits H, Ploegh H (1986) Monoclonal antibodies raised against denatured HLA-B locus heavy chains permit biochemical characterization of certain HLA-C locus products. J Immunol 137(7):2299–2306
Perosa F, Luccarelli G, Prete M, Favoino E, Ferrone S, Dammacco F (2003) {beta}2-microglobulin-free HLA class I heavy chain epitope mimicry by monoclonal antibody HC-10-specific peptide. J Immunol 171(4):1918–1926
Sernee MF, Ploegh HL, Schust DJ (1998) Why certain antibodies cross-react with HLA-A and HLA-G: epitope mapping of two common MHC class I reagents. Mol Immunol 35(3):177–188
Maio M, Altomonte M, Tatake R, Zeff RA, Ferrone S (1991) Reduction in susceptibility to natural killer cell-mediated lysis of human FO-1 melanoma cells after induction of HLA class I antigen expression by transfection with B2m gene. J Clin Investig 88(1):282–289
Bandoh N, Ogino T, Cho HS, Hur SY, Shen J, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S (2005) Development and characterization of human constitutive proteasome and immunoproteasome subunit-specific monoclonal antibodies. Tissue Antigens 66(3):185–194. doi:10.1111/j.1399-0039.2005.00462.x
Wang X, Campoli M, Cho HS, Ogino T, Bandoh N, Shen J, Hur SY, Kageshita T, Ferrone S (2005) A method to generate antigen-specific mAb capable of staining formalin-fixed, paraffin-embedded tissue sections. J Immunol Methods 299(1–2):139–151
Ogino T, Wang X, Kato S, Miyokawa N, Harabuchi Y, Ferrone S (2003) Endoplasmic reticulum chaperone-specific monoclonal antibodies for flow cytometry and immunohistochemical staining. Tissue Antigens 62:385–393. doi:10.1034/j.1399-0039.2003.00114.x
Fujita M, Zhu X, Sasaki K, Ueda R, Low KL, Pollack IF, Okada H (2008) Inhibition of STAT3 promotes the efficacy of adoptive transfer therapy using type-1 CTLs by modulation of the immunological microenvironment in a murine intracranial glioma. J Immunol 180(4):2089–2098
Racanelli V, Leone P, Frassanito MA, Brunetti C, Perosa F, Ferrone S, Dammacco F (2010) Alterations in the antigen processing-presenting machinery of transformed plasma cells are associated with reduced recognition by CD8+ T cells and characterize the progression of MGUS to multiple myeloma. Blood 115(6):1185–1193. doi:10.1182/blood-2009-06-228676
Zhai Y, Yang JC, Spiess P, Nishimura MI, Overwijk WW, Roberts B, Restifo NP, Rosenberg SA (1997) Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother 20(1):15–25
le Doussal V, Tubiana-Hulin M, Hacene K, Friedman S, Brunet M (1989) Nuclear characteristics as indicators of prognosis in node negative breast cancer patients. Breast Cancer Res Treat 14(2):207–216. doi:10.1007/bf01810737
Atkins D, Breuckmann A, Schmahl GE, Binner P, Ferrone S, Krummenauer F, Störkel S, Seliger B (2004) MHC class I antigen processing pathway defects, ras mutations and disease stage in colorectal carcinoma. Int J Cancer 109(2):265–273. doi:10.1002/ijc.11681
Bandoh N, Ogino T, Katayama A, Takahara M, Katada A, Hayashi T, Harabuchi Y (2010) HLA class I antigen and transporter associated with antigen processing downregulation in metastatic lesions of head and neck squamous cell carcinoma as a marker of poor prognosis. Oncol Rep 23(4):933–939
Kloetzel P-M (2004) The proteasome and MHC class I antigen processing. Biochimica et Biophysica Acta (BBA) Mol Cell Res 1695(1–3):225–233
Hansen TH, Bouvier M (2009) MHC class I antigen presentation: learning from viral evasion strategies. Nat Rev Immunol 9(7):503–513
Seliger B (2008) Molecular mechanisms of MHC class I abnormalities and APM components in human tumors. Cancer Immunol Immunother 57(11):1719–1726. doi:10.1007/s00262-008-0515-4
Mehling M, Simon P, Mittelbronn M, Meyermann R, Ferrone S, Weller M, Wiendl H (2007) WHO grade associated downregulation of MHC class I antigen-processing machinery components in human astrocytomas: does it reflect a potential immune escape mechanism? Acta Neuropathol 114(2):111–119. doi:10.1007/s00401-007-0231-8
Lopez-Albaitero A, Nayak JV, Ogino T, Machandia A, Gooding W, DeLeo AB, Ferrone S, Ferris RL (2006) Role of antigen-processing machinery in the in vitro resistance of squamous cell carcinoma of the head and neck cells to recognition by CTL. J Immunol 176(6):3402–3409
Lou Y, Vitalis TZ, Basha G, Cai B, Chen SS, Choi KB, Jeffries AP, Elliott WM, Atkins D, Seliger B, Jefferies WA (2005) Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific immune responses and survival. Cancer Res 65(17):7926–7933. doi:10.1158/0008-5472.can-04-3977
Lou Y, Basha G, Seipp RP, Cai B, Chen SS, Moise AR, Jeffries AP, Gopaul RS, Vitalis TZ, Jefferies WA (2008) Combining the antigen processing components TAP and tapasin elicits enhanced tumor-free survival. Clin Cancer Res 14(5):1494–1501. doi:10.1158/1078-0432.ccr-07-1066
Sarrabayrouse G, Corvaisier M, Ouisse L-H, Bossard C, Mével BL, Potiron L, Meurette G, Gervois N, Jotereau F (2011) Tumor-reactive CD4+CD8αβ+ CD103+ αβT cells: a prevalent tumor-reactive T-cell subset in metastatic colorectal cancers. Int J Cancer 128(12):2923–2932. doi:10.1002/ijc.25640
Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA, Lin YG, Merritt WM, Spannuth WA, Deavers MT, De Geest K, Gershenson DM, Lutgendorf SK, Ferrone S, Sood AK (2008) HLA class I antigen processing machinery component expression and intratumoral T-cell infiltrate as independent prognostic markers in ovarian carcinoma. Clin Cancer Res 14(11):3372–3379. doi:10.1158/1078-0432.ccr-07-4433
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964
Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, Parsa AT (2010) CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci 17(11):1381–1385
Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C (2011) Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res 17(13):4296–4308. doi:10.1158/1078-0432.CCR-10-2557
He H, Somlo G, Yun Y, Chu PG (2009) Dendritic cell infiltration in lymph node positive breast carcinomas. Breast J 15(2):218–220. doi:10.1111/j.1524-4741.2009.00707.x
Mansfield A, Heikkila P, von Smitten K, Vakkila J, Leidenius M (2011) Metastasis to sentinel lymph nodes in breast cancer is associated with maturation arrest of dendritic cells and poor co-localization of dendritic cells and CD8+ T cells. Virchows Arch 459(4):391–398. doi:10.1007/s00428-011-1145-3
Zhang J, Patel L, Pienta KJ (2010) CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev 21(1):41–48. doi:10.1016/j.cytogfr.2009.11.009
Mantovani A, Savino B, Locati M, Zammataro L, Allavena P, Bonecchi R (2010) The chemokine system in cancer biology and therapy. Cytokine Growth Factor Rev 21(1):27–39. doi:10.1016/j.cytogfr.2009.11.007
Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc P-H, Trajanoski Z, Fridman W-H, Galon J (2005) Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med 353(25):2654–2666. doi:10.1056/NEJMoa051424
Lott ST, Chen N, Chandler DS, Yang Q, Wang L, Rodriguez M, Xie H, Balasenthil S, Buchholz TA, Sahin AA, Chaung K, Zhang B, Olufemi S-E, Chen J, Adams H, Band V, El-Naggar AK, Frazier ML, Keyomarsi K, Hunt KK, Sen S, Haffty B, Hewitt SM, Krahe R, Killary AM (2009) DEAR1 is a dominant regulator of acinar morphogenesis and an independent predictor of local recurrence-free survival in early-onset breast cancer. PLoS Med 6(5):e1000068
Varga D, Koenig J, Kuhr K, Strunz K, Geyer V, Kurzeder C, Atassi Z, Blettner M, Kreienberg R, Woeckel A (2010) Comparison of early onset breast cancer patients to older premenopausal breast cancer patients. Arch Gynecol Obstet 282(4):427–432. doi:10.1007/s00404-009-1339-y
Bauer K, Parise C, Caggiano V (2010) Use of ER/PR/HER2 subtypes in conjunction with the 2007 St Gallen Consensus Statement for early breast cancer. BMC Cancer 10(1):228
Van Belle V, Van Calster B, Brouckaert O, Vanden Bempt I, Pintens S, Harvey V, Murray P, Naume B, Wiedswang G, Paridaens R, Moerman P, Amant F, Leunen K, Smeets A, Drijkoningen M, Wildiers H, Christiaens M-R, Vergote I, Van Huffel S, Neven P (2010) Qualitative assessment of the progesterone receptor and HER2 improves the Nottingham Prognostic Index up to 5 years after breast cancer diagnosis. J Clin Oncol 28(27):4129–4134. doi:10.1200/jco.2009.26.4200
Dunnwald L, Rossing M, Li C (2007) Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res 9(1):R6
Sari E, Guler G, Hayran M, Gullu I, Altundag K, Ozisik Y (2011) Comparative study of the immunohistochemical detection of hormone receptor status and HER-2 expression in primary and paired recurrent/metastatic lesions of patients with breast cancer. Med Oncol 28(1):57–63. doi:10.1007/s12032-010-9418-2
Gong Y, Han EY, Guo M, Pusztai L, Sneige N (2011) Stability of estrogen receptor status in breast carcinoma. Cancer 117(4):705–713. doi:10.1002/cncr.25506
Herrmann F, Lehr H-A, Drexler I, Sutter G, Hengstler J, Wollscheid U, Seliger B (2004) HER-2/neu-mediated regulation of components of the MHC class I antigen-processing pathway. Cancer Res 64(1):215–220. doi:10.1158/0008-5472.can-2522-2
Mimura K, Ando T, Poschke I, Mougiakakos D, Johansson CC, Ichikawa J, Okita R, Nishimura MI, Handke D, Krug N, Choudhury A, Seliger B, Kiessling R (2011) T cell recognition of HLA-A2 restricted tumor antigens is impaired by the oncogene HER2. Int J Cancer 128(2):390–401. doi:10.1002/ijc.25613
Acknowledgments
Lindsay Mock and Louise Mazur for collecting tissue slides and clinical data; Xiaojuan Deng for antibody preparation; Mitsugu Fujita for technical assistance. The Musella Foundation for Brain Tumor Research & Information (HO); The Walter L. Copeland Fund of the Pittsburgh Foundation (YL); NIH/NCI 1P01 CA132714 (HO); NIH/NINDS 2P01 NS40923 (HO); This project used the UPCI Animal Facility and was supported in part by award P30CA047904.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
262_2011_1137_MOESM1_ESM.pptx
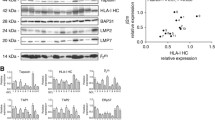
Expression of HLA class I heavy chain, tapasin and LMP2 expression in primary and brain metastasis of breast cancer. (A) Representative immunohistochemical (IHC) staining on primary breast cancer tissues (left) and metastatic brain lesions (right). (B) Comparing the expression level HLA class I heavy chain, tapasin and LMP2 in primary breast cancer cases without known brain metastases (Primary, N = 50) and unpaired metastatic brain lesions (Metastatic, N = 33) (PPTX 1596 kb)
262_2011_1137_MOESM2_ESM.pptx
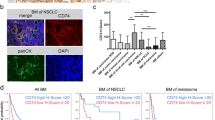
Absence of associations between the extent of CD8+ T cell infiltration and expression levels of HLA class I heavy chain, β2 microglobulin, tapasin, TAP2 or LMP2. Data were analyzed by Kendall’s tau-b test (PPTX 61 kb)
Rights and permissions
About this article
Cite this article
Liu, Y., Komohara, Y., Domenick, N. et al. Expression of antigen processing and presenting molecules in brain metastasis of breast cancer. Cancer Immunol Immunother 61, 789–801 (2012). https://doi.org/10.1007/s00262-011-1137-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-011-1137-9