Abstract
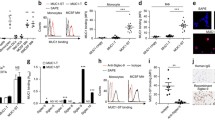
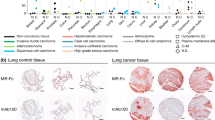
The epithelial mucin MUC1 is a high molecular weight membrane glycoprotein frequently overexpressed and aberrantly glycosylated in adenocarcinoma. Mucins normally contain high amounts of O-linked carbohydrate structures that may influence immune reactions to this antigen. During malignant transformation, certain glyco-epitopes of MUC1, such as Tn-antigen, TF-antigen and their sialylated forms become exposed. The role of these glycan structures in tumor biology is unknown, but their presence is known to correlate with poor prognosis in several adenocarcinomas. We analyzed the potency of MUC1 containing Tn-antigens (MUC1-Tn) to target C-type lectins that function as carbohydrate recognition and uptake molecules on dendritic cells (DC). We identified the macrophage galactose type C-type lectin (MGL), expressed by both DC and macrophages, as the receptor for recognition and binding of MUC1-Tn. To validate the occurrence of MGL–MUC1 interactions in situ, we studied the binding of MGL to MUC1 in primary colon carcinoma tissue. Isolation of MUC1 out of colon carcinoma tissue showed strong binding activity to MGL. Interestingly, MGL binding to MUC1 was highly correlated to binding by the lectin Helix pomatia agglutinin (HPA), which is associated with poor prognosis in colorectal cancer. The detection of MGL positive cells in situ at the tumor site together with the modified glycosylation status of MUC1 to target MGL on DC suggests that MGL positive antigen presenting cells may play a role in tumor progression.








Similar content being viewed by others
References
Baeckstrom D, Hansson GC, Nilsson O, Johansson C, Gendler SJ, Lindholm L (1991) Purification and characterization of a membrane-bound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins. J Biol Chem 266:21537–21547
Gendler SJ, Burchell JM, Duhig T, Lamport D, White R, Parker M, Taylorpapadimitriou J (1987) Cloning of partial cdna-encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. PNAS 84:6060–6064
Lan MS, Batra SK, Qi WN, Metzgar RS, Hollingsworth MA (1990) Cloning and sequencing of a human pancreatic tumor mucin cdna. J Biol Chem 265:15294–15299
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer protection and control of the cell surface. Nat Rev Cancer 4:45–60
Agrawal B, Krantz MJ, Parker J, Longenecker BM (1998) Expression of MUC1 mucin on activated human T cells: implications for a role of MUC1 in normal immune regulation. Cancer Res 58:4079–4081
Cloosen S, Thio M, Vanclee A, van Leeuwen EB, Senden-Gijsbers BL, Oving EB, Germeraad WT, Bos GM (2004) Mucin-1 is expressed on dendritic cells, both in vitro and in vivo. Int Immunol 16:1561–1571
Wykes M, MacDonald KP, Tran M, Quin RJ, Xing PX, Gendler SJ, Hart DN, McGuckin MA (2003) MUC1 epithelial mucin (CD227) is expressed by activated dendritic cells. J Leukoc Biol 72:692–701
Correa I, Plunkett T, Vlad A, Mungul A, Candelora-Kettel J, Burchell JM, Taylor-Papadimitriou J, Finn OJ (2003) Form and pattern of MUC1 expression on T cells activated in vivo or in vitro suggests a function in T-cell migration. Immunology 108:32–41
Parry S, Hanisch FG, Leir SH, Sutton-Smith M, Morris HR, Dell A, Harris A (2006) N-glycosylation of the MUC1 mucin in epithelial cells and secretions. Glycobiology 16:623–634
Nakamori S, Ota DM, Cleary KR, Shirotani K, Irimura T (1994) MUC1 mucin expression as a marker of progression and metastasis of human colorectal carcinoma. Gastroenterology 106:353–361
Boland CR, Deshmukh GD (1990) The carbohydrate composition of mucin in colonic cancer. Gastroenterology 98:1170–1177
Dalziel M, Whitehouse C, McFarlane I, Brockhausen I, Gschmeissner S, Schwientek T, Clausen H, Burchell JM, Taylor-Papadimitriou J (2001) The relative activities of the C2GnT1 and ST3Gal-I glycosyltransferases determine O-glycan structure and expression of a tumor-associated epitope on MUC1. J Biol Chem 276:11007–11015
Hanisch FG, Uhlenbruck G, Peter-Katalinic J, Egge H, Dabrowski J, Dabrowski U (1989) Structures of neutral O-linked polylactosaminoglycans on human skim milk mucins. A novel type of linearly extended poly-N-acetyllactosamine backbones with Gal beta(1–4)GlcNAc beta(1–6) repeating units. J Biol Chem 264:872–883
Hanisch FG, Stadie TR, Deutzmann F, Peter-Katalinic J (1996) MUC1 glycoforms in breast cancer—cell line T47D as a model for carcinoma-associated alterations of 0-glycosylation. Eur J Biochem 236:318–327
Lloyd KO, Burchell J, Kudryashov V, Yin BW, Taylor-Papadimitriou J (1996) Comparison of O-linked carbohydrate chains in MUC-1 mucin from normal breast epithelial cell lines and breast carcinoma cell lines. Demonstration of simpler and fewer glycan chains in tumor cells. J Biol Chem 271:33325–33334
Cao Y, Stosiek P, Springer GF, Karsten U (1996) Thomsen–Friedenreich-related carbohydrate antigens in normal adult human tissues: a systematic and comparative study. Histochem Cell Biol 106:197–207
Itzkowitz SH, Yuan M, Montgomery CK, Kjeldsen T, Takahashi HK, Bigbee WL, Kim YS (1989) Expression of Tn, sialosyl-Tn, and T antigens in human colon cancer. Cancer Res 49:197–204
Schumacher U, Higgs D, Loizidou M, Pickering R, Leathem A, Taylor I (1994) Helix pomatia agglutinin binding is a useful prognostic indicator in colorectal carcinoma. Cancer 74:3104–3107
Brooks SA, Leathem AJ (1991) Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet 338:71–74
Kakeji Y, Tsujitani S, Mori M, Maehara Y, Sugimachi K (1991) Helix pomatia agglutinin binding activity is a predictor of survival time for patients with gastric carcinoma. Cancer 68:2438–2442
Figdor CG, van Kooyk Y, Adema GJ (2002) C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev 2:77–84
Geijtenbeek TB, van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM, Appelmelk B, van Kooyk Y (2003) Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 197:7–17
Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M, Laskarin G, Monti P, Piemonti L, Biondi A, Mantovani A, Introna M, Allavena P (2003) Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 171:4552–4560
Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM (2002) Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med 196:1627–38
Steinman RM, Hawiger D, Liu K, Bonifaz L, Bonnyay D, Mahnke K, Iyoda T, Ravetch J, Dhodapkar M, Inaba K, Nussenzweig M (2003) Dendritic cell function in vivo during the steady state: a role in peripheral tolerance. Immune Mech Dis 987:15–25
van Gisbergen KPJM, Aarnoudse CA, Meijer GA, Geijtenbeek TBH, van Kooyk Y (2005) Dendritic cells recognize tumor-specific glycosylation of carcinoembryonic antigen on colorectal cancer cells through dendritic cell-specific intercellular adhesion molecule-3-grabbing nonintegrin. Cancer Res 65:5935–5944
Mungul A, Cooper L, Brockhausen I, Ryder K, Mandel U, Clausen H, Rughetti A, Miles DW, Taylor-Papadimitriou J, Burchell JM (2004) Sialylated core 1 based O-linked glycans enhance the growth rate of mammary carcinoma cells in MUC1 transgenic mice. Int J Oncol 25:937–943
Higashi N, Fujioka K, Denda-Nagai K, Hashimoto S, Nagai S, Sato T, Fujita Y, Morikawa A, Tsuiji M, Miyata-Takeuchi M, Sano Y, Suzuki N, Yamamoto K, Matsushima K, Irimura T (2002) The macrophage C-type lectin specific for galactose/N-acetylgalactosamine is an endocytic receptor expressed on monocyte-derived immature dendritic cells. J Biol Chem 277:20686–20693
van Vliet SJ, van Liempt E, Saeland E, Aarnoudse CA, Appelmelk B, Irimura T, Geijtenbeek TB, Blixt O, Alvarez R, van DI, van Kooyk Y (2005) Carbohydrate profiling reveals a distinctive role for the C-type lectin MGL in the recognition of helminth parasites and tumor antigens by dendritic cells. Int Immunol 17:661–669
Sallusto F, Lanzavecchia A (1994) Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 179:1109–1118
Backstrom M, Link T, Olson FJ, Karlsson H, Graham R, Picco G, Burchell J, Taylor-Papadimitriou J, Noll T, Hansson GC (2003) Recombinant MUC1 mucin with a breast cancer-like O-glycosylation produced in large amounts in Chinese-hamster ovary cells. Biochem J 376:677–686
Stanley P (1989) Chinese-hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cel Biol 9:377–383
Geijtenbeek TBH, Torensma R, van Vliet SJ, van Duijnhoven GCF, Adema GJ, van Kooyk Y, Figdor CG (2000) Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575–585
Geijtenbeek TBH, van Kooyk Y, van Vliet SJ, Renes MH, Raymakers RA, Figdor CG (1999) High frequency of adhesion defects in B-lineage acute lymphoblastic leukemia. Blood 94:754–764
Van Die I, van Vliet SJ, Kwame Nyame A, Cummings RD, Bank CM, Appelmelk B, Geijtenbeek TB, van Kooyk Y (2003) The dendritic cell specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis-X. Glycobiology 13:471–478
Iida S, Yamamoto K, Irimura T (1999) Interaction of human macrophage C-type lectin with O-linked N-acetylgalactosamine residues on mucin glycopeptides. J Biol Chem 274:10697–10705
Jerome KR, Barnd DL, Bendt KM, Boyer CM, Taylor-Papadimitriou J, McKenzie IF, Bast RC Jr, Finn OJ (1991) Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res 51:2908–2916
Kotera Y, Fontenot JD, Pecher G, Metzgar RS, Finn OJ (1994) Humoral immunity against a tandem repeat epitope of human mucin MUC-1 in sera from breast, pancreatic, and colon cancer patients. Cancer Res 54:2856–2860
Mensdorff-Pouilly S, Verstraeten AA, Kenemans P, Snijdewint FG, Kok A, van Kamp GJ, Paul MA, Van Diest PJ, Meijer S, Hilgers J (2000) Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol 18:574–583
Suzuki N, Yamamoto K, Toyoshima S, Osawa T, Irimura T (1996) Molecular cloning and expression of cDNA encoding human macrophage C-type lectin. Its unique carbohydrate binding specificity for Tn antigen. J Immunol 156:128–135
Hiltbold EM, Vlad AM, Ciborowski P, Watkins SC, Finn OJ (2000) The mechanism of unresponsiveness to circulating tumor antigen MUC1 is a block in intracellular sorting and processing by dendritic cells. J Immunol 165:3730–3741
Dwek MV, Ross HA, Streets AJ, Brooks SA, Adam E, Titcomb A, Woodside JV, Schumacher U, Leathem AJ (2001) Helix pomatia agglutinin lectin-binding oligosaccharides of aggressive breast cancer. Int J Cancer 95:79–85
Laack E, Nikbakht H, Peters A, Kugler C, Jasiewicz Y, Edler L, Hossfeld DK, Schumacher U (2002) Lectin histochemistry of resected adenocarcinoma of the lung: helix pomatia agglutinin binding is an independent prognostic factor. Am J Pathol 160:1001–1008
Steinman RM, Nussenzweig MC (2002) Avoiding horror autotoxicus: The importance of dendritic cells in peripheral T cell tolerance. PNAS 99:351–358
Zhang K, Baeckstrom D, Brevinge H, Hansson GC (1996) Secreted MUC1 mucins lacking their cytoplasmic part and carrying sialyl-Lewis a and x epitopes from a tumor cell line and sera of colon carcinoma patients can inhibit HL-60 leukocyte adhesion to E-selectin-expressing endothelial cells. J Cell Biochem 60:538–549
Zhang K, Sikut R, Hansson GC (1997) A MUC1 mucin secreted from a colon carcinoma cell line inhibits target cell lysis by natural killer cells. Cell Immunol 176:158–165
Van Vliet SJ, van Liempt E, Geijtenbeek TBH, van Kooyk Y (2006) Differential regulation of C-type lectin expression on tolerogenic dendritic cell subsets. Immunobiology 211:577–585
Acknowledgements
The authors thank Dr. Juan-Jesus Garcia-Vallejo and Dr. Marjolein van Egmond, VU University Medical Center, Amsterdam, for helpful discussions. ES and MB were supported by the European Union Grant QLK3-CT-2002-01980, MB was also supported by QLK3-CT-2002-02010 and by the Swedish Research Council, The Swedish Cancer Foundation, Assar Gabrielsson’s foundation for cancer research, Lars Hierta’s foundation and Magn Bervall’s foundation. SV and VCMvdB were supported by an NWO Pioner Grant 900-02-002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeland, E., van Vliet, S.J., Bäckström, M. et al. The C-type lectin MGL expressed by dendritic cells detects glycan changes on MUC1 in colon carcinoma. Cancer Immunol Immunother 56, 1225–1236 (2007). https://doi.org/10.1007/s00262-006-0274-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-006-0274-z