Abstract
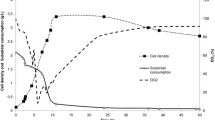
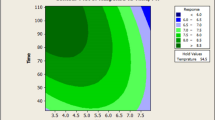
Bacillus subtilis spore preparations are promising probiotics and biocontrol agents, which can be used in plants, animals, and humans. The aim of this work was to optimize the nutritional conditions using a statistical approach for the production of B. subtilis (WHK-Z12) spores. Our preliminary experiments show that corn starch, corn flour, and wheat bran were the best carbon sources. Using Plackett–Burman design, corn steep liquor, soybean flour, and yeast extract were found to be the best nitrogen source ingredients for enhancing spore production and were studied for further optimization using central composite design. The key medium components in our optimization medium were 16.18 g/l of corn steep liquor, 17.53 g/l of soybean flour, and 8.14 g/l of yeast extract. The improved medium produced spores as high as \( 1.52 \pm 0.06 \times {10^{10}}{\text{spores}}/{\text{ml}} \) under flask cultivation conditions, and \( 1.56 \pm 0.07 \times {10^{10}}{\text{spores}}/{\text{ml}} \) could be achieved in a 30-l fermenter after 40 h of cultivation. To the best of our knowledge, these results compared favorably to the documented spore yields produced by B. subtilis strains.


Similar content being viewed by others
References
Box GEP, Wilson KB (1951) On the experimental attainment of optimum conditions. J Roy Stat Soc B 13:1–45
Brückner H, Langer M, Lupke M, Westhauser T, Godel H (1995) Liquid chromatographic determination of amino acid enantiomers by derivatization with o-phthaldialdehyde and chiral thiols applications with reference to food science. J Chromatogr A 697:229–245
Brütickner H, Westhauser T (1994) Chromatographic determination of d-amino acids as native constituents of vegetables and fruits. Chromatographia 39:419–426
Cartman ST, La Ragione RM (2004) Spore probotics as animal feed supplements. In: Ricca E, Henriques AO, Cutting SM (eds) Bacterial spores: probiotics and emerging applications. Horizon Science, London, pp 155–161
Cazemier AE, Wagenaars SFM, Ter Steeg PF (2001) Effect of sporulation and recovery medium on the heat resistance and amount of injury of spores from Bacilli. J Appl Microbiol 90:761–770
Chang YN, Huang JC, Lee CC, Shih LL, Tzeng YM (2002) Use of response surface methodology to optimize culture medium for production of lovastatin by Monascus rubber. Enzyme Microb Tech 30:889–894
Chen QH, He GQ, Mokhtar AMA (2002) Optimization of medium composition for the production of elastase by Bacillus sp EL31410 with response surface methodology. Enzyme Microb Tech 30:667–672
Cochran WG, Cox GM (1957) Experiment designs, 2nd edn. Wiley, New York
le Duc H, Hong HA, Barbosa TM, Henriques AO, Cutting SM (2004) Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 70:2161–2171
Emmert EAB, Handelsman J (1999) Biocontrol of plant disease—a (Gram-) positive perspective. FEMS Microbiol Lett 171:1–9
Erbe T, Brückner H (2000) Chromatographic determination of amino acid enantiomers in beers and raw materials used for their manufacture. J Chromatogr A 881:81–91
Hageman JH, Shankweiler GW, Wall PR, Franich K, McCowan GW, Cauble SM, Grajeda J, Quinones C (1984) Single, chemically defined sporulation medium for Bacillus subtilis growth, sporulation, and extracellular protease production. J Bacteriol 160:438–441
Hartley HO (1959) Smallest composite designs for quadratic response surfaces. Biometrics 15:611–624
Heck JX, Flores SH, Hertzm PF, Ayub MAZ (2005) Optimization of cellulase-free xylanase activity produced by Bacillus coagulans BL69 in solid-state cultivation. Process Biochem 40:107–112
Hong HA, le Duc H, Cutting SM (2005) The use of bacterial spore formers as probiotics. FEMS Mcrobiol Rev 29:813–835
Kang BC, Lee SY, Chang HN (1992) Enhanced spore production of Bacillus thuringiensis by fed-batch culture. Biotechnol Lett 14:721–726
Kennedy M, Krouse D (1999) Strategies for improving fermentation medium performance: a review. J Ind Microbiol Biotechnol 23:456–475
Liu JG, Xing JM, Chang TS, Ma ZY, Liu HZ (2005) Optimization of nutritional conditions for nattokinase production by Bacillus natto NLSSE using statistical experimental methods. Process Biochem 40:2757–2762
Luna CL, Mariano RLR, Souto-Maior AM (2002) Production of a biocontrol agent for crucifers black rot disease. Brazilian J Chem Eng 19:133–140
Luna CL, Silva GR, Rios EMMM (2004) Bacillus thuringiensis var. israelensis production involving re-use of the supernatant. Biotechnol Lett 26:143–145
Molin G, Svensson M (1976) Formation of dry-heat resistant Bacillus subtilis var. niger spores as influenced by the composition of the sporulation medium. Antonie Van Leeuwenhoek 42:387–395
Monteiro SM, Clemente JJ, Henriques AO, Gomes RJ, Carrondo MJ, Cunha AE (2005) A procedure for high-yield spore production by Bacillus subtilis. Biotechnol Prog 21:1026–1031
Montesinos E (2003) Development, registration and commercialization of microbial pesticides for plant protection. Int Microbiol 6:245–252
Nagórska K, Biokowsk M, Obuchowski M (2007) Multicellular behaviour and production of a wide variety of toxic substances support usage of Bacillus subtilis as a powerful biocontrol. Acta Biochim Pol 54:1–14
Paik H, Lee K, Kim J, Jun S, Kim W (2001) Optimization for culture medium and fermentation process of a Bacillus probiotics. Abstr Gen Meet Am Soc Microbiol 101:549–550
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33:305–325
Pryor Scott W, Gibson Donna M, Hay Anthony G, Gossett James M, Walker Larry P (2007) Optimization of spore and antifungal lipopeptide production during the solid-state fermentation of Bacillus subtilis. Appl Biochem Biotechnol 143:63–79
Sasaki K, Jiaviriyaboonya S, Rogers PL (1998) Enhancement of sporulation production by corn steep liquor feeding during intermittent fed-batch culture of Bacillus sphaericus2362. Biotechnol Lett 20:165–168
Sella SRBR, Dlugokenski REF, Guizelini BP, Vandenberghe LPS, Medeiros ABP, Pandey A, Soccol CR (2008) Selection and optimization of Bacillus atrophaeus inoculum medium and its effect on spore yield and thermal resistance. Appl Biochem Biotechnol 151:380–392
Senesi S (2004) Bacillus spores as probiotics products for human use. In: Ricca E, Henriques AO, Cutting SM (eds) Bacterial spores: probiotics and emerging applications. Horizon, London, pp 132–141
Shi FY, Zhu YB (2006) Application of statistically-based experimental designs in medium optimization for spore production of Bacillus subtilis from distillery effluent. BioControl 52:845–853 30
Shoda M (2000) Bacterial control of plant diseases. J Biosci Bioeng 89:515–521
Ushakova NA (2004) Two-stage process of cultivation of probiotic Bacillus subtilis 8130 for reception biologically active feed additives for animals. In: Zaikov GE (ed) Biotechnology and industry.Nova, Commack, NY
Van Handel E (1985) Rapid determination of glycogen and sugars in mosquitoes. J Am Mosq Control Assoc 1:299–301
Vasantha N, Freese E (1979) The role of manganese in growth and sporulation of Bacillus subtilis. J Gen Microbiol 112:329–336
Xiao ZJ, Liu PH, Qin JY, Xu P (2007) Statistical optimization of medium components for enhanced acetoin production from molasses and soybean meal hydrolysate. Appl Microbiol Biotechnol 74:61–68
Zhao Shumiao Hu, Nan HJ, Yunxiang L, Bin Z (2008) High-yield spore production from Bacillus licheniformis by solid state fermentation. Biotechnol Lett 30:295–297
Acknowledgments
We thank Dr. Jian-Ping Liu (Max Planck Institute for Infection Biology, Berlin, Germany) and Dr. Barry Wong (Wuhan University, P. R. China) for their advice and critical reading of the manuscript. This work was funded by the National Natural Sciences Foundation of China (NSFC Grants No.30770042).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Table S1
Independent variables studied in Plackett–Burman screening design (DOC 35 kb)
Table S2
Plackett–Burman design for the ten independent variables in coded values and measured responses (DOC 55.5 kb)
Table S3
Regression analysis of Plackett–Burman design for spore production (DOC 33 kb)
Table S4
The design and results of the path of steepest ascent experiment (DOC 29 kb)
Table S5
Coded and actual values for the independent variables in the central composite design (DOC 30 kb)
Table S6
Optimization of spore yields and experimental results for central composite design (CCD; DOC 40 kb)
Table S7
Regression analysis of central composite design in B. subtilis (WHK-Z12)spore production (DOC 33 kb)
Table S8
Analysis of variance for the model fitting spore yields (DOC 31 kb)
Table S9
Ridge analysis for the maximal spore yields of B. subtilis WHK-Z12 (DOC 40 kb)
Rights and permissions
About this article
Cite this article
Chen, ZM., Li, Q., Liu, HM. et al. Greater enhancement of Bacillus subtilis spore yields in submerged cultures by optimization of medium composition through statistical experimental designs. Appl Microbiol Biotechnol 85, 1353–1360 (2010). https://doi.org/10.1007/s00253-009-2162-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2162-x